A detailed characterization of platinum-cerium-alumina model catalysts under Water Gas Shift conditions have been performed.
 In the water gas shift (WGS) reaction, CO reacts with steam producing $ \rm CO_2 $ and $ \rm H_2 $. It is an important reaction to decrease the CO level in the syngas, deriving from the process of steam reforming of natural gas or other sources. In addition, WGS is one of the main reactions to produce $ \mathbf{H_2} $ at industrial scale. In that case, it is performed in two steps: the first one at high temperature, with iron-based catalysts, and the second one at low temperature, with copper-based catalysts.
In the water gas shift (WGS) reaction, CO reacts with steam producing $ \rm CO_2 $ and $ \rm H_2 $. It is an important reaction to decrease the CO level in the syngas, deriving from the process of steam reforming of natural gas or other sources. In addition, WGS is one of the main reactions to produce $ \mathbf{H_2} $ at industrial scale. In that case, it is performed in two steps: the first one at high temperature, with iron-based catalysts, and the second one at low temperature, with copper-based catalysts.
Recently, the development of proton exchange membrane fuel cell technology, which requires $ \rm H_2$ with low CO levels, has boosted the search for new WGS catalysts. Platinum is a non-pyrophoric WGS catalyst with high activity but the stability of Pt nanoparticles and their activity depends on the nature of the support. One of the most studied supports is ceria. Thirty years ago, changes on ceria redox behaviour caused by the interaction with platinum were demonstrated. Later, several studies pointed out that Pt is anchored on $ \rm CeO_2$ through Pt-O-Ce bond, improving Pt stability and its catalytic properties.
Cerium has a low redox potential (measure of the tendency to acquire electrons and be reduced), changing its oxidation state from $ \rm Ce^{4+}$ to $ \rm Ce^{5+}$ and leading to the formation of non-stoichiometric oxides $ \rm CeO_{2-x}$ with capacity to store and release oxygen dependent on the atmosphere. It has been shown that several aspects such as crystallographic orientation in cerium oxide, particle size, presence of doping atoms ($ \rm Ti^{4+}$, $ \rm La^{3+}$, $ \rm Zr^{4+}$, etc.), presence of alkali metals, among others, affect the formation of oxygen vacancies, the interaction with gases and/or the interaction with metallic particles affecting its catalytic performance.
Nevertheless, one important aspect that has not been deeply studied is the direct impact of the structural characteristics of nm-sized ceria phase in the overall activity. Despite extensive work, some issues about WGS on Pt-ceria remain under intense debate:
Two main mechanisms have been discussed: redox and associative. The redox pathway involves the participation of O atoms from the support; while in the associative mechanism the O is provided by $ \rm H_2O$ activation. Variants such as associative with redox regeneration involving OH species generated with the O from the support have been proposed. The intermediaries such as formates, carboxylates, carbonates, that are formed in the associative pathway have also been debated. It seems now consensus that a unified mechanism for all Pt catalysts does not exist, depending on the Pt size, support and interaction as well as on the catalytic conditions.
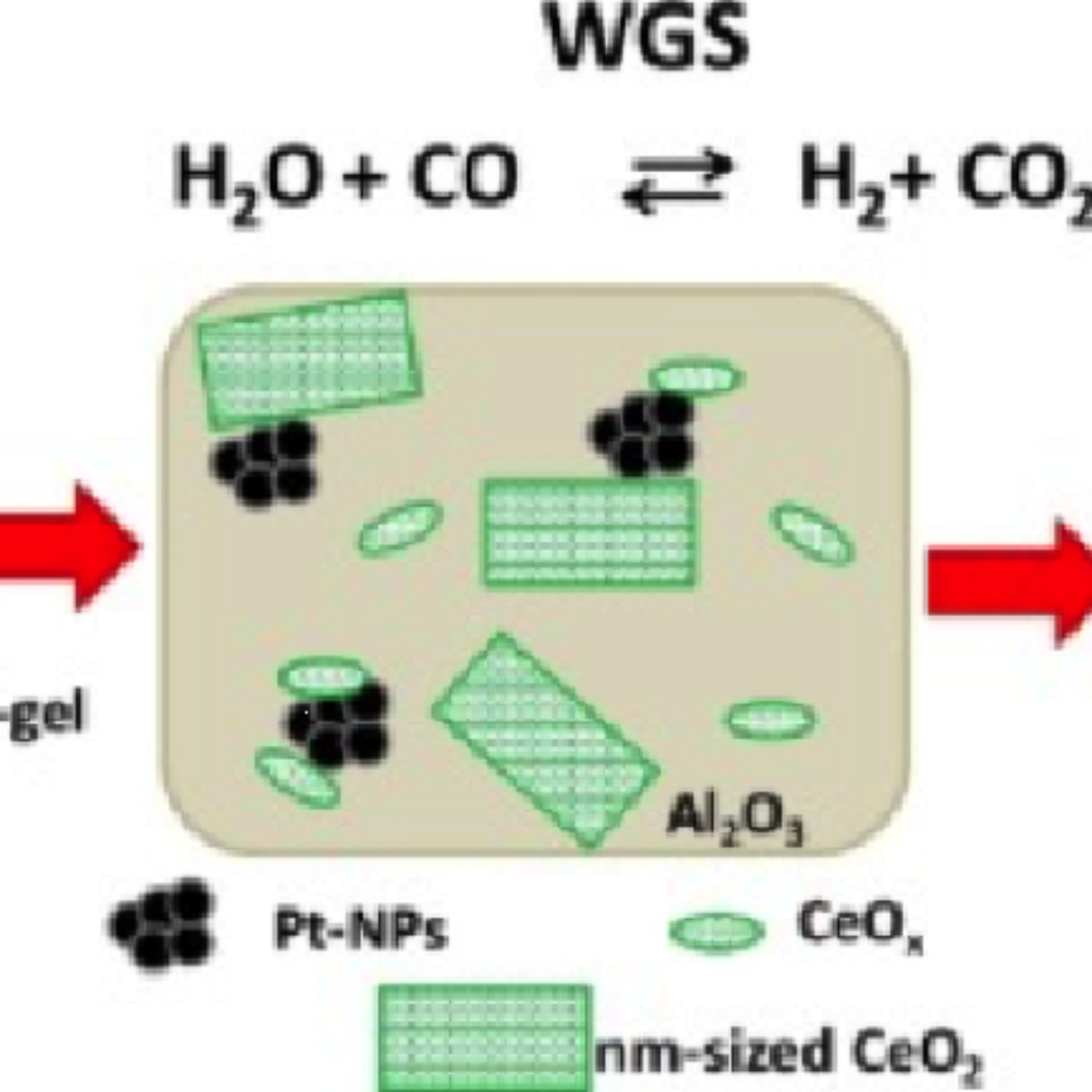
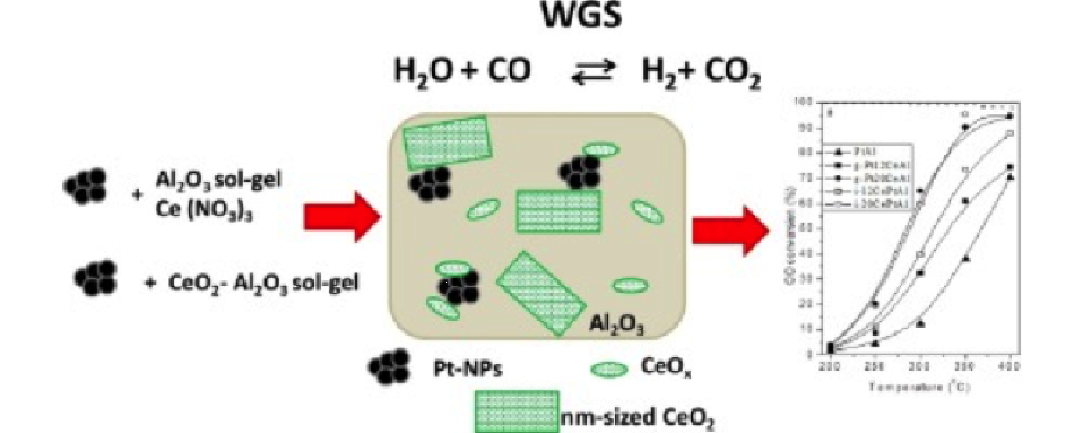
In a recent work, D. Meira et al. [1], have performed a detailed characterization of platinum-cerium-alumina model catalysts under WGS conditions by using in situ techniques and pre-formed platinum nanoparticles (Pt-NPs), which were encapsulated in $ \rm Al_2O_3$ sol-gel modified with $ \rm CeO_2$ or encapsulated in $ \rm CeO_2-Al_2O_3$ sol-gel. The prepared catalysts were characterized by various experimental techniques: X-Ray Diffraction, Infrared Spectroscopy, X-Ray Photoemission (XPS) done at the LNLS, while in situ X-Ray Absorption Spectroscopy experiments were performed at the ESRF [Pt L3 edge (11564 eV) and Ce K edge (40443 eV)] and at the XAFS1 beamline in LNLS [Ce L3 edge (5723 eV)]. Then WGS reactions were carried out in a fixed bed quartz reactor under atmospheric pressure. The results show a lack of direct dependence of the activity with the average ceria structural properties suggesting that the creation of the interfacial Pt-O-Ce sites is likely to be the limiting factor in these samples. The presence of Ce in PtAl catalysts increases the WGS specific reaction rates up to 7 times in the WGS reaction. Several parameters affect the activity and the use of preformed Pt-NPs was the strategy adopted to prepare model systems focusing on the support and interface effects. It was possible to highlight differences and similarities among the samples concerning several parameters related to the cerium oxide phase, such as ceria crystalline domain size and local environment, the initial contact between the metal and the oxide, the ceria oxidation state among others. However, a clear identification of a possible main ceria parameter governing the reaction turned out to be challenging and a direct correlation between the properties that were observed by both ex situ and in situ experiments and the catalyst activity could not be clearly made. The main parameter affecting activity seems to be ceria loading: however, the increase in specific reaction rate values due to the presence of ceria was significantly smaller than predicted by theory considering a Pt-O-Ce interface.
These results suggest that the odds to create the true active sites might be the limiting factor in these samples and that they are related to the contact to highly dispersed Ce-Ox species. Nevertheless, the mechanism of how different species of ceria (such as highly dispersed CeOx species in contact with alumina and few nm-sized crystallites of ceria) operate in water activation and intermediates formation has still to be deeper understood.
Source:
[1] D.M. Meira, R.U. Ribeiro, O. Mathon, S. Pascarelli, J.M.C. Bueno and D. Zanchet, Complex interplay of structural and surface properties of ceria onplatinum supported catalyst under water gas shift reaction. (2016) Applied Catalysis B: Environmental, 197, pp. 73-85. DOI: 10.1016/j.apcatb.2016.04.025
Results suggest that ionic liquid hybrid organosilica/Pd-NPs under multiphase conditions operate akin to catalytically active membranes.
The proposed process could offer important technological and environmental advantages.