Research investigates method for the production of protein crystals, based on thin films organized by an external electric field
Once a disease-related protein is identified as a therapeutic target, the study of its three-dimensional structure – the positions of each of its atoms and their interactions – allows a deep understanding of its action in the body, and its interaction with a potential drug. In this way, it is possible to discover potential new drugs, or to understand the functioning of known drugs and to increase their effectiveness.
Protein crystallography is an essential tool in the investigation of the three-dimensional structure of these molecules and, consequently, of their biological function. So much so that about 90% of the currently known protein structures were determined using X-ray diffraction in protein crystals. However, in order to apply this technique, it is necessary to obtain a protein crystal of adequate quality, which requires quite specific conditions.
The limiting step in this process is nucleation, which requires orderly agglomeration of protein molecules to initiate crystal growth. There are several methods to facilitate such nucleation (and growth) of protein crystals. However most of these methods still have limitations: many do not bother to evaluate the quality of formed crystals and others involve the use of sophisticated technologies.
In a recent study published in the journal ACS Omega [1], Tássia Karina Walter et al. presented an alternative method for protein crystallization.
As a model, the researchers used lysozyme, an enzyme with antimicrobial function abundant in secretions such as tears, saliva and mucus. This molecule is capable of breaking down components of the cell wall of bacteria, causing their death. Lysozyme was the first protein to have its three-dimensional structure elucidated by X-ray diffraction methods.
In their new method, the researchers used as support for the crystal formation a thin film constituted by the protein itself, previously prepared and subjected to an electrical potential difference.
The application of the potential difference results in the orientation of the protein molecules in the film, as verified by atomic force microscopy images. The preparation of the film does not require sophisticated equipment and can be easily carried out in any molecular biology laboratory.
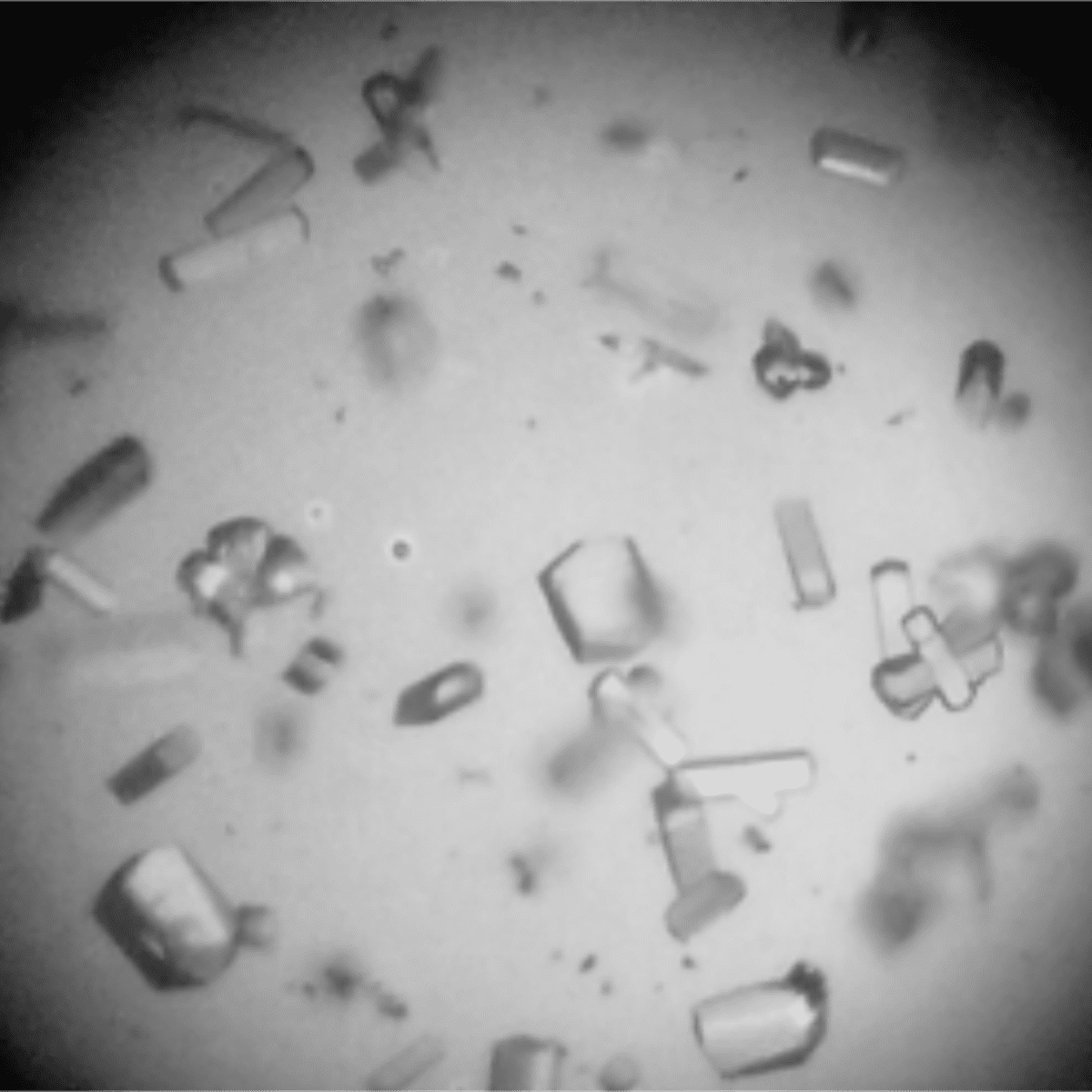
These films, later used as support for the growth of crystals under different conditions, allowed an increase in the nucleation rate, that is, a larger number of crystals in a shorter incubation time. In some cases, they have also resulted in the formation of larger crystals with more regular morphology, which allows better data collection by X-ray diffraction. In addition, the use of these films indicates the possibility of obtaining crystals in conditions closer to the activity (which would allow the study of its structure in its active form), whereas in the classical method more extreme conditions are usually employed.
X-ray diffraction experiments were performed in the MX2 Macromolecular Crystallography beamline of the Brazilian Synchrotron Light Laboratory (LNLS). Through the analysis of the data, it was possible to evaluate the quality of the crystals obtained from the thin films and to compare it with the classical method, used as control.
In some cases, the crystals showed diffraction properties similar to the control, which demonstrates that even with increasing nucleation speed it is possible to collect diffraction data with good quality. In other cases, it was possible to significantly increase the resolution limit of the crystals in relation to the control, which allows for more information in the construction of models of the protein structure.
Thus, by using these films of organized proteins as a support for crystallization, several possibilities are opened in the study of the three-dimensional structure of proteins, including the obtaining of high quality crystals in conditions closer to their functional role, using very accessible resources.
Source: [1] Tássia Karina Walter, Cecília Fabiana da Gama Ferreira, Jorge Iulek, and Elaine Machado Benelli. Use of Protein Thin Film Organized by External Electric Field as a Template for Protein Crystallization. ACS Omega 2018 3 (8), 8683-8690. DOI: 10.1021/acsomega.8b01277
Research determines markers to identify those responsible for the emission of iron-rich particles in cities
Research investigates reproducing the morphology of complex biological systems in the nanoscale for technological purposes