CONTACT & STAFF
Facility Tel.: +55 19 3517 5170
Facility E-mail: manaca@lnls.br
Coordination: Andrey F. Z. Nascimento
Tel.: +55 19 3518 2325
E-mail: andrey.nascimento@lnls.br
Click here for more information on this Facility team.
MANACÁ – Macromolecular Micro and Nano Crystallography – is a fourth-generation beamline at the Brazilian Synchrotron Light Laboratory (LNLS-Sirius) dedicated to the determination of structures of biological macromolecules and small molecules using conventional X-ray crystallography. The beamline team is expanding its capabilities toward state-of-the-art methods of protein crystallography, such as room temperature (RT) and serial crystallography (SSX). We have developed and implemented a fixed target pipeline approach with a 3D-printed sample holder, which is optimized for sample crystallization, handling, and data collection of multi-crystals. Support for data analysis in both conventional and serial crystallography is available to users.
The experimental station has an automatic sample changer that can assemble a sample in less than 1 minute, allowing data collection of more than 30 samples per shift. Configurations are being prepared for the collection and analysis of serial crystallography data, as well as automation and remote collection procedures. Proposals must be submitted via SAU ONLINE portal and usage dates can be scheduled by email manaca@lnls.br.
Facility Tel.: +55 19 3517 5170
Facility E-mail: manaca@lnls.br
Coordination: Andrey F. Z. Nascimento
Tel.: +55 19 3518 2325
E-mail: andrey.nascimento@lnls.br
Click here for more information on this Facility team.
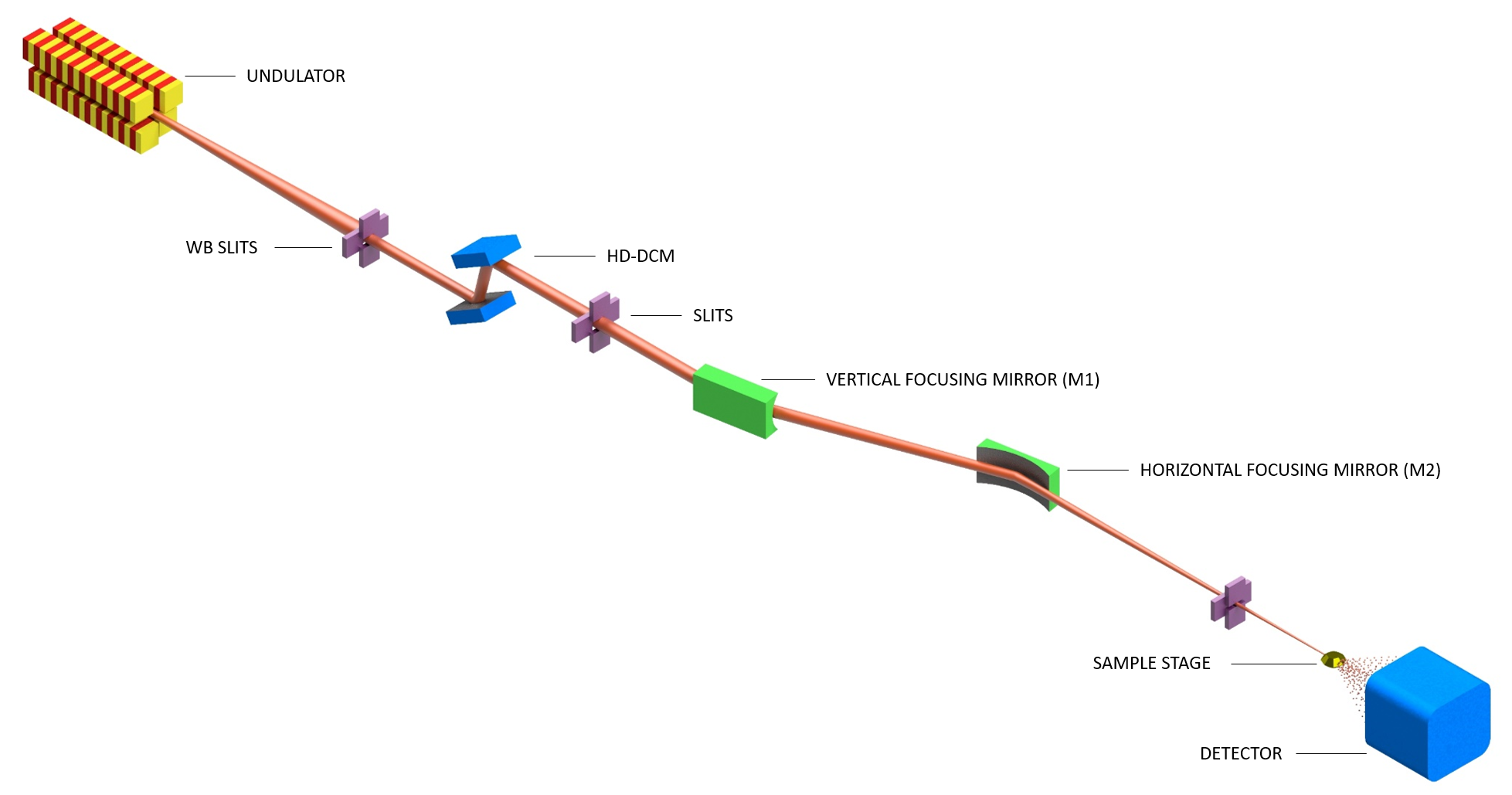
MANACÁ beamline employs two X-ray mirrors as focusing optics. In the optical hutch, the first optical element of the beamline is a horizontal Double-Crystal Monochromator (DCM) equipped with two pairs of Si crystals (111 and 311), followed by the vertical focusing mirror (side-bounce sagittal cylindrical mirror). The horizontal focusing mirror (tangential elliptical mirror) is in the experimental hutch microMANACA. This system currently provides a beam cross-section varying from 20×20 to 80×100 µm² (FWHM). This optical configuration allows for varying the beam size at the sample position by slightly changing the mirrors incidence angle, a great feature for matching beam and crystal size, and is optimized for high flux.
Tunable beam sizes at the focal point are achieved by small angular offsets on the mirrors M1 and M2, and a small lateral dislocation of the experimental hutch table.
The microManacá experimental station hutch houses the M2 mirror, attenuators, sample stage, detector and sample delivery system. The sample stage granite table holds the slits, shutter, airbearing based goniometer, beamstopper, shutter, beam monitoring devices and on-axis video microscope (Arinax, Inc). A metallic support holds the cryo-cooler system, fluorescence detector, and other elements to be defined in the future, such as a humidifier for room temperature experiments. A separate table holds the automated sample delivery system.
| Parameter | Value | Condition |
|---|---|---|
| Energy range | 5 – 20 keV | – |
| Energy resolution (ΔE/E) | 10-4 | – |
| Harmonic content | <10-4 | – |
| Energy scan | Yes | – |
| Beam size | 0.5×0.5 μm 10×7 to 100×80 μm |
nano station micro station |
| Beam divergence | < 0.5 mrad | – |
Beamline setups for conventional (oscillation) cryogenic mode are available at the MANACÁ beamline to on-site and/or remotely data collection using an automatic sample changer with experiment control made by MXCuBE3 and automatic data processing pipeline (from data reduction to initial phasing).
The Manacá beamline now offers an automated sample changer, capable of holding 48 pins in a Nitrogen filled dewar. The samples should be frozen in pins SPINE “MiTeGen B5” and “MiTeGen B5-R” and “Hampton SPINE HT”.
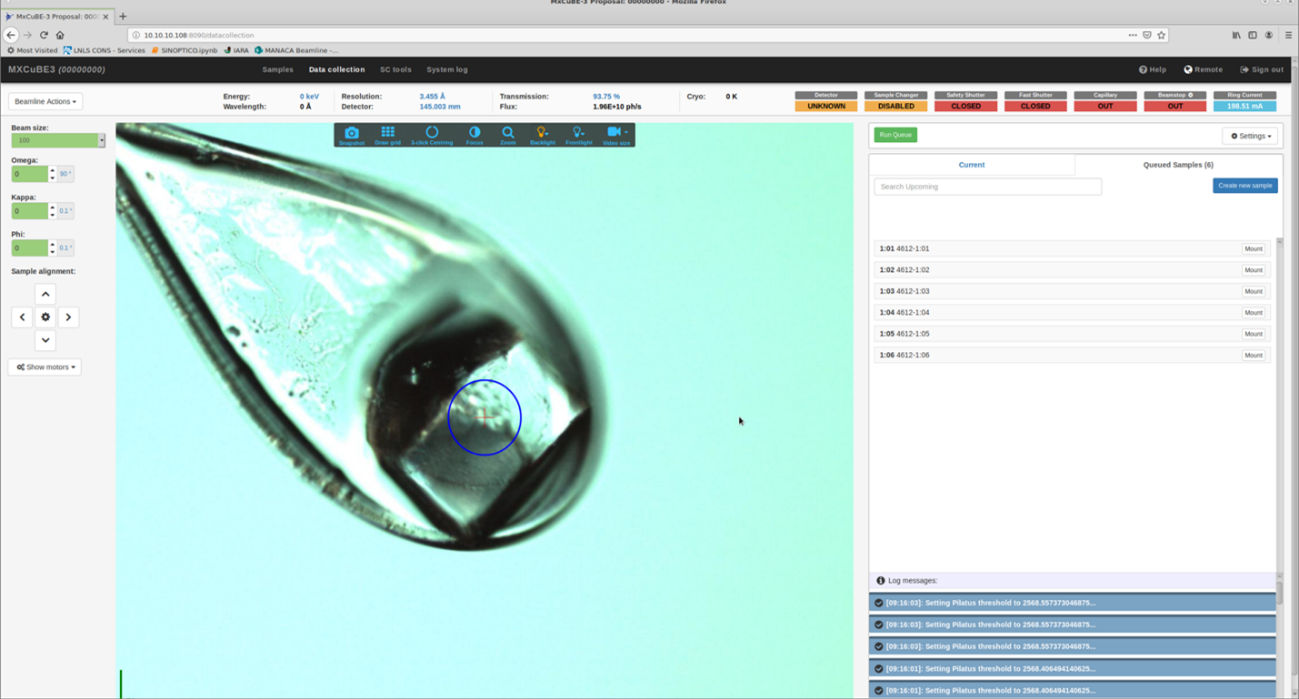
The robotic arm transfers the sample to the fixed goniometer and the nitrogen flow resumes. The user can access a live view of a camera focused on the sample, as well as the images from the sample microscope BZOOM, through the MXCube interface.
The robotic arm is sent to pick a sample pin from the dewar after checking that there is no other sample in the goniometer base; the arm picks up a sample and delivers it to the sample holder. We then see the view of the sample through the on-axis microscope, via the MXCube interface.
We are part of the MXCUBE software development community, and currently employ MXCube3, web version, which can be accessed remotely and allows use of the beamline interfaces.
In parallel with data collection, users can access a series of software pipelines to treat the data and analyze the quality of the experiment as soon as it is complete. Currently a dataset can be acquired in about 6 minutes.
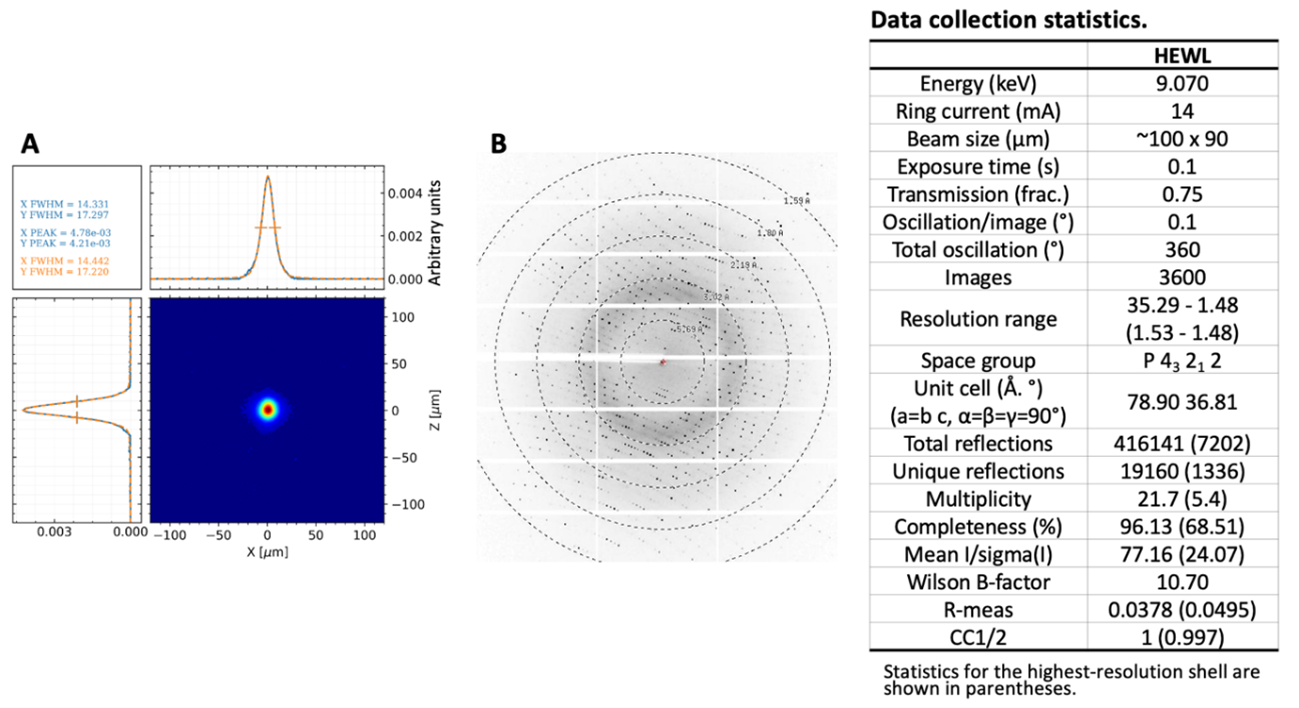
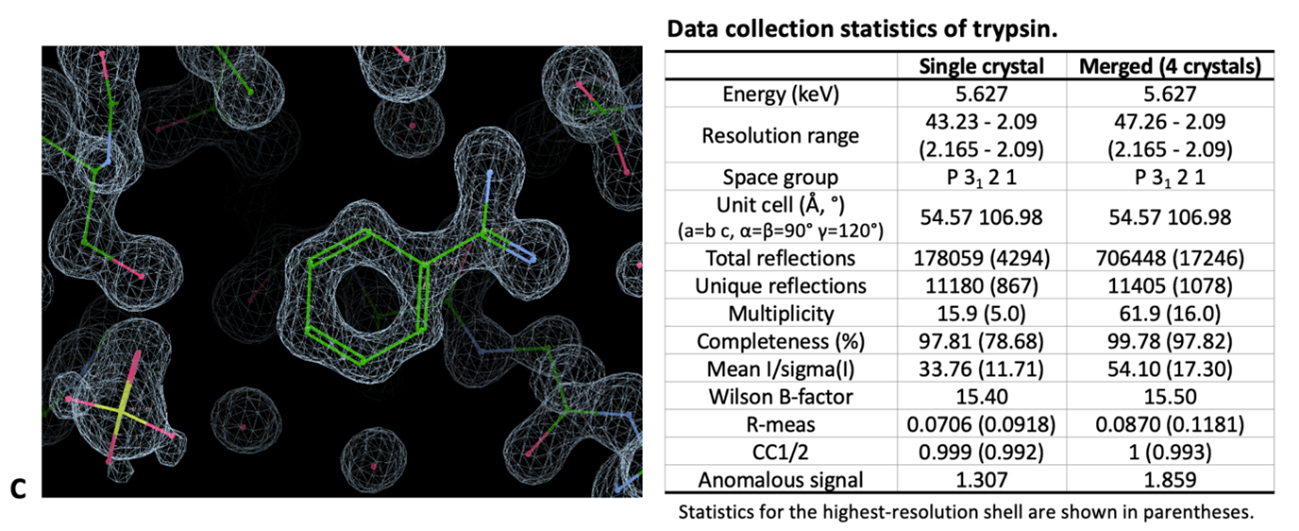
Several strategies are being implemented to collect multi-crystal/serial room-temperature and time-resolved data, ranging from sample collection devices to software for data treatment
The Manacá beamline now offers an automated sample changer, capable of holding 48 pins in a Nitrogen filled dewar. The samples should be frozen in pins SPINE 18mm in Unipucks.
Several strategies are being implemented to collect multi-crystal/serial room-temperature and time-resolved data, ranging from sample collection devices to software for data treatment.
Protein crystals can be prepared at the ROBOLAB facility at LNBio, accessible via SAU ONLINE.
The possibility of determining the three-dimensional structure of proteins, that is, the positions of each of the atoms and their interactions, has allowed the development of new technologies for drug discovery and became one of the most striking examples of advances brought by synchrotrons. In fact, today there are over 150000 known protein structures recorded in the Protein Data Bank (PDB), about 80% of which were resolved with the use of synchrotron radiation. The Protein Data Bank already holds several structures collected at LNLS, and some already collected at the MANACA beamline employing Fragment Based Drug Discovery strategies (FBDD).
The rational drug design strategy, for example, attempts to identify opportunities to block or modify these molecular interactions of proteins. By identifying a protein as the target for a certain therapy, the structural studies with synchrotron radiation can show how drugs bind to a protein, identify structural modifications to improve the drug binding, or even suggest which modifications in their molecular structure can be made without affecting its binding capabilities.
Some of the earliest successful examples of this approach, wherein the structure guided rational design of drugs, include Captopril (Capoten) for treating hypertension by inhibiting angiotensin converting enzymes; Dorzolamide (Trusopt) for the treatment of glaucoma by the inhibition of carbonic anhydrase; also many of the drugs that are part of anti-AIDS cocktails which inhibit HIV protease; and drugs Zanamivir (Relenza) and Oseltamivir (Tamiflu) to treat influenza type A and influenza type B, also much used as a treatment during the pandemic of swine flu (H1N1) in 2009. The latter inhibit a surface protein of influenza virus, neuraminidase. In the treatment of some cancers, inflammatory diseases and diabetes, this rational design has been used widely by the so-called kinases. They constitute a class of enzymes, which actively participate in the regulation of cellular processes such as metabolism, growth, and differentiation. Several kinase inhibitors developed by rational design have been the subjects of clinical trials, being the most successful Imatinib (Gleevec). This drug is used to treat chronic myeloid leukemia, gastrointestinal stromal tumors, and many other malignancies. It is also worth mentioning that this was the first generic medication for cancer treatment to be produced in Brazil. All these drugs were created by a combination of clinical insight factors, chemical, and biological tests and had synchrotron crystallography as a decisive tool to elucidate the microscopic mechanisms to lead drugs to reach their targets.
Regarding beam size, micron and sub-micron beams are paramount to match the X-ray beam more accurately to the crystal size in challenging (but frequently encountered) experiments with small crystals, and to probe small, but more uniform, regions of a larger but less uniform crystal. The size of the crystals used for X-ray crystallography is of particular importance because the integrated intensity of an X-ray diffraction peak from a crystal is proportional to the ratio of its diffracting volume to its unit cell volume. The larger and more complicated the protein structure becomes, the more challenging are the tasks required to isolate the protein intact and to grow large, well-diffracting crystals. Membrane proteins are notoriously difficult to crystallize due to their amphiphilic nature.
Concerning samples that are more sensitive to radiation damage, the small beams allow to raster across the crystal to expose fresh sample to X-rays throughout the collection of a data set. Additionally, there is evidence that a large fraction of the damage caused by the impinging radiation is caused by photoelectrons produced near the X-ray beam. These photoelectrons are preferentially ejected along the polarization vector and are more likely to deposit their energy near the exposed spot. By keeping the beam size sufficiently small, the exposed portion of the sample remains less damaged than its surrounding.
The MANACÁ beamline is dedicated to conventional MX experiments and also to the development of room temperature (RT) and synchrotron serial crystallography (SSX) methodologies, to be available to the local and worldwide user communities of structural biology. The strategy includes the production and test of a set of standard microfluidics-based devices, data processing pipelines with a user friendly and efficient environment, further development of protocols, and tools to make fixed-target and in-flow delivery approaches reliable as user-friendly routines at the MANACÁ beamline. The beamline team focus and ongoing work is geared towards the developing standard and novel crystallographic devices, methodologies for rapid data collection, processing, and analysis of RT and SSX, including fixed target and in-flow sample delivery systems (future). The fixed target systems are powerful tools in RT and serial crystallography data collection. They will be dedicated to single and multi-crystal data collection of micro-to-nano-sized protein crystals at cryogenic and/or room temperature conditions. In-flow sample delivery systems will be dedicated to elucidating protein structural dynamics by detecting transient intermediate states in protein-ligand interactions. Microfluidic and high-viscosity injector-based systems are the two types of in-flow systems that will be available for users.
We are currently working on the commissioning and developing goniometer/non-goniometer-based microfluidic devices dedicated to high-viscosity media injection and mix-and-injecting studies. It should enable structural biologists to access serial crystallographic techniques and room temperature data collection at the microMANACÁ station. Initial native SAD experiments were conducted at 6.6 keV, where a Sodium (Na), ion could be detected between the side chain atoms. We are using devices created at Professors Alke Meents and Richard Neutze laboratories and have been conducting fruitful exchanges of ideas. This approach favors the large-scale testing of ligands and the analysis of ligand-inducing plasticity in structural studies (protein-ligand screening), mapping enzyme intermediates and structural changes induced by chemical (i.e., ligands, cofactors, etc.) and physical (i.e., light, pressure, etc.) factors at cryogenic and/or room temperature conditions.