Researchers look for substitutes extracted from sunflower oil
The ingestion of lipids, oils and fats, provides essential ingredients to our metabolism. The worries about several cardiovascular diseases caused by the excessive consumption of saturated fats and cholesterol from animal origin, though, have prompted the development of alternative sources of lipids.
Among those, there are the hydrogenated vegetable fats, which, for being from vegetal origin, are free from cholesterol. Yet, they contain the so-called trans fats, considered not only to cause the rising of LDL – the “bad cholesterol” – but also for causing the lowering of HDL – the “good cholesterol” – levels in the organism. That way, the use of trans fats is under severe restriction and even prohibition in countries around the world.
In this context, scientists from Universidad de Buenos Aires and Universidad Nacional de San Martín in Argentine, and Utah State University, in the USA, used the experimental facilities for x-ray scattering in the Brazilian Synchrotron Light Laboratory (LNLS). They investigated the properties of fats extracted from a special kind of sunflower oil, as substitute to trans fats in industrialized food products.
Of oils and fats
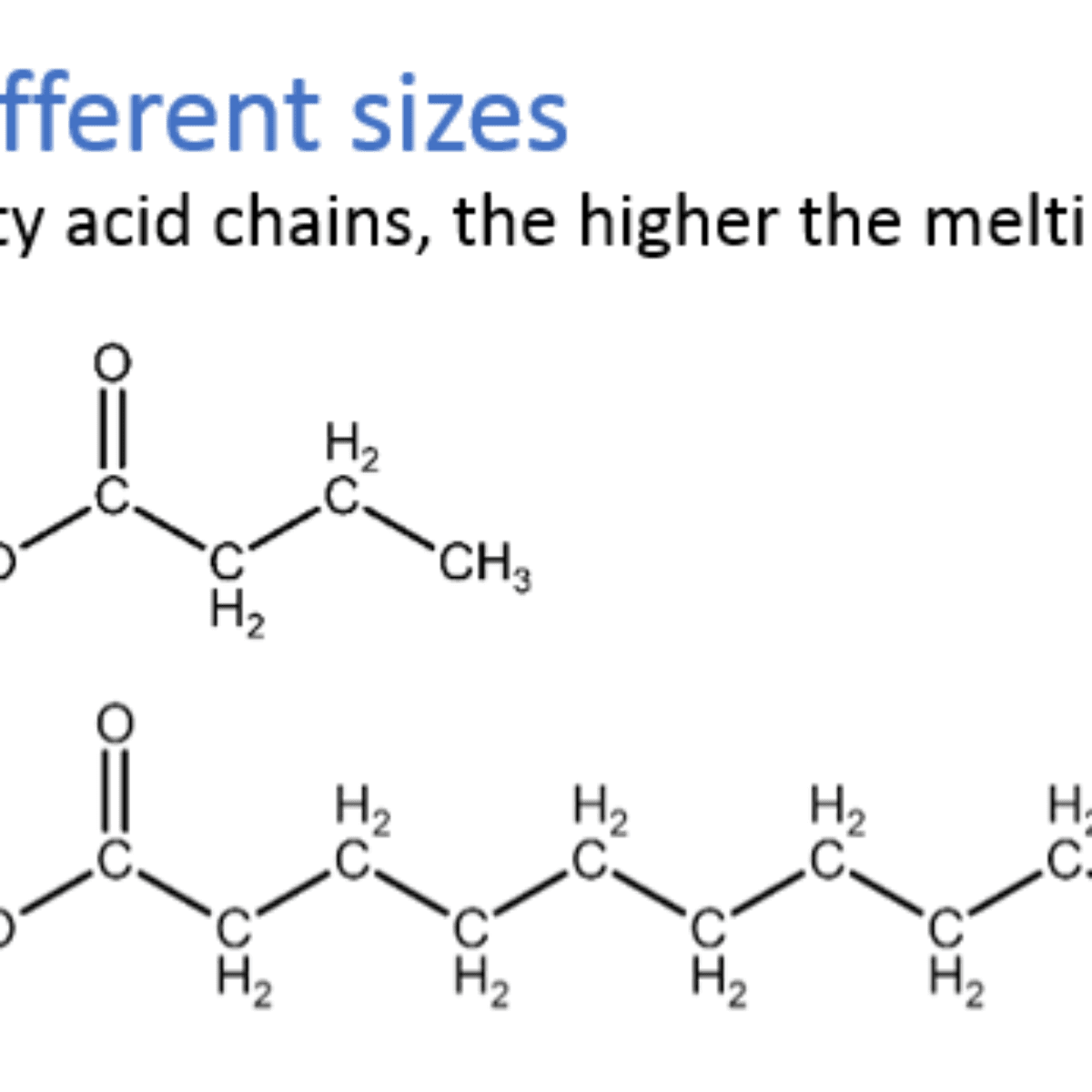
Both oils and fats are made of complex mixtures of substances called triacylglycerols: organic molecules formed from one glycerol and three fatty acid molecules.
The difference lies in the physical at which they are found at room temperature due to their molecular structure. In general, oils are liquid due to shorter carbon chains while fats are solid for having longer carbon chains.
Besides that, the amount of unsaturation – the number of double bonds between carbon atoms in the carbon chain is also an influence in the physical state of lipids. Mono- or polyunsaturated lipids have lower melting points than saturated lipids with carbon chains of same size.
In addition to that, unsaturated fatty acid molecules can be found in two different geometric configurations: cis and trans, which indicate the relative position between carbon chains in a double carbon bond. Molecules in the cis configuration lead to lipids that are less resistant to changes in temperature and thus have a lower melting point than lipids containing trans isomers.



From oil to fat
Lipids extracted from plants are usually found as oils. Since many applications in the food industry require the use of fats, there are several chemical and physical processes to obtain one from the other.
The cheapest and most famous process is the hydrogenation: a chemical process in which unsaturated oils react with hydrogen, with the help from a catalyzer, and have the double bonds in their carbon chains reorganized into simple bonds. It produces partially or totally saturated lipids, which have higher melting point [and are usually solid].
Trans fats are a side product of the hydrogenation process. They are very important to the food industry for presenting not only great thermal stability and malleability, but also resistance to oxidation, which greatly enhances the shelf life of products. In spite of that, the discovery of the health risks associated to consumption of trans fats brings upon the industry an ever growing pressure to abandon its use.
In Brazil, although there are no plans for prohibition or obligatory restriction in the use of trans fats, an agreement was made, in 2007, between the Ministry of Health and the Brazilian Food Industry Association (ABIA). The goal was for the industries to voluntarily reduce the amount of trans fats to a maximum of 5% of the total fat content in processed food, and 2% in oils and margarines. By 2011, the industries achieved 96.4% of those objectives.
European countries have also been establishing strict limits for the use of trans fats. Additionally, in the USA, the Food and Drug Administration (FDA) has decided to no longer consider trans fats to be safe for human consumption and the food industry in the country has until 2018 to comply to the new regulations by finding new sources of fats appropriate to their use.
Another way of extracting fats from vegetable oils – without producing trans fats – is the physical process known as fractionation, which separates the liquid lipids – called oleins – from the solid lipids – called stearins.
Through the techniques of dry and solvent fractionation, the scientists extracted two kinds of stearins from a high-stearic high-oleic sunflower oil (HSHOSFO) produced from non-GMO sunflower seeds cultivated in the province of Buenos Aires, in Argentine.
Fats and figures
Solid fats organize themselves in a mixture of crystalline configurations, called polymorphisms. The geometry of each different configuration in which the fats can be found, called polymorphic phase or form, defines their macroscopic properties such as color and texture.
When the fats are not in the right form for a given industrial application, they must go through physical processes to rearrange their crystalline structure. Upon heating, the bonds between fat molecules are broken and the crystals are destroyed. When cooled, the fats crystalize back into different polymorphic forms. The challenge lies in obtaining only, or mainly one of those polymorphic forms.
With that in mind, the scientists put through different heating and cooling cycles each of the stearins extracted from the sunflower oil to observe the formation of different polymorphs. To follow closely on the crystallization process, they used the Small-angle X-ray Scattering (SAXS) technique in the SAXS1 beamline from the Brazilian Synchrotron Light Laboratory. According to the corresponding scientist, Maria Lidia Herrera, “SAXS is a better choice if the material has not high crystallinity. The only way to follow polymorphic transition of a fat in real time is using a synchrotron source, since scattering patterns may be acquired in 10 seconds. These experiments cannot be performed with a copper target x-ray source.”
That way, they managed to identify the proper thermal treatment for obtaining specific polymorphic forms for each stearin extracted from the HSHOSFO sunflower oil, two of which are important for bakery and confectionary products. The scientists hope their work represent a valuable contribution to the search for substitutes to trans fats.
Source:
Learning about its optical properties
Research can lead to new treatments against envenoming