Encapsulation and addition of excipients were demonstrated to be promising
Lactose is the main carbohydrate present in milk. To be digested and absorbed by the body, it must be broken down into its constituents: glucose and galactose. The breakdown is catalyzed by an enzyme called lactase, produced in the small intestine of young mammals especially for the digestion of the maternal milk.
The production of this enzyme decreases with time, making the body progressively incapable of digesting lactose. Thus, despite the social habit of consuming milk from other animals and dairy products after childhood, around 65% of the world population has some degree of lactose intolerance.
The symptoms of this intolerance are mainly gastrointestinal, including abdominal pain, bloating and diarrhea, which manifest between 30 minutes and two hours after the ingestion of dairy products. To avoid those symptoms, intolerant people need either to ingest the enzyme lactose together with dairy products, or to consume lactose-free industrialized products that have had the enzyme added during their manufacture.
However, the breakdown of milk sugar is not only beneficial for intolerant people, but also for the enhancement of food properties. Lactose has a tendency, for example, to retain odors, cause the hardening of powdered products due to the absorption of water and even impair the texture of ice creams due to its crystallization. This makes lactase an enzyme widely used in the food industry. In spite of this, this enzyme has low stability when subjected to thermal and mechanical treatments, which reduces its efficiency in industrial processes.
Thus, researchers from the Universidad Politécnica de Valencia (Spain) and Universidad de Buenos Aires (Argentina) used the facilities of the Brazilian Synchrotron Light Laboratory (LNLS) to investigate the encapsulation of lactase in millimetric beads of Calcium Alginate as a method for the conservation of its enzymatic activity upon thermal treatments.
Alginate is a low cost polymer extracted from algae. It is highly biocompatible and resistant to contamination, and widely used in the food, cosmetic and pharmaceutical industries. Alginate is used as a thickener, as emulsifier and foam stabilizer, and for the encapsulation of substances. However, in terms of enzyme protection, calcium alginate beads show low resistance to physical treatments, such as heating and dehydration, causing losses in enzymatic activity.
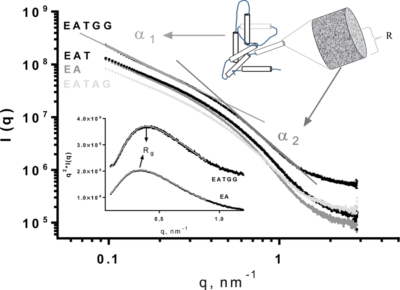
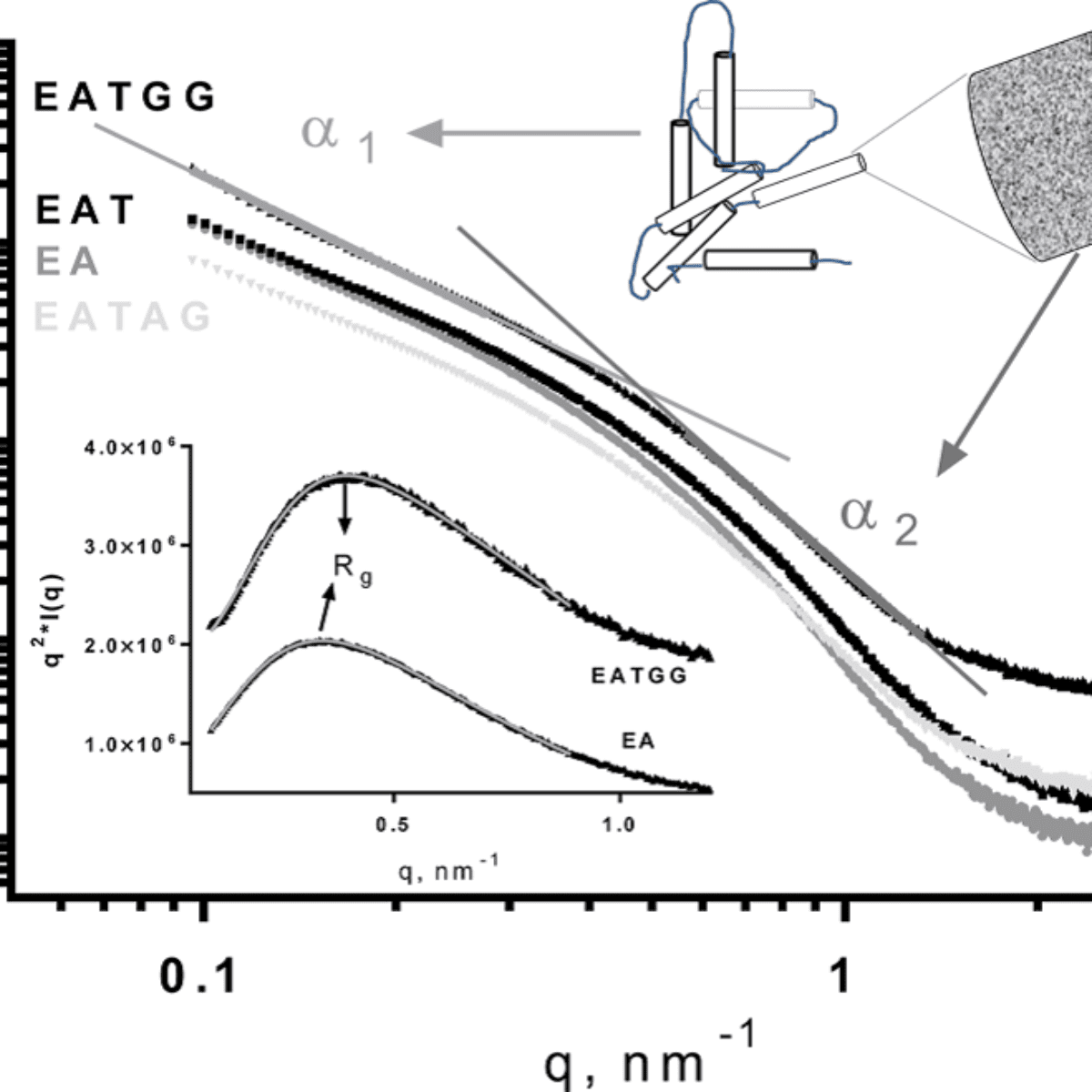
SAXS profiles of Calcium Alginate beads (A) containing the enzyme lactase (E) with addition of Trehalose (T), Arabic Gum (AG) and Guar Gum (GG).
To improve these properties, the researchers added other excipients to the alginate beads (Trehalose, Arabic Gum and Guar Gum, all extensively used in the food industry) and investigated the effect on the microstructure and thermal properties.
According to the researchers, the addition of excipients reduced the recovery of the enzyme in all cases, with losses between 20% and 30%. However, the inclusion of Trehalose was critical for the conservation of the enzyme during the freezing and freezing/thawing treatments. Furthermore, the presence of Guar Gum increased the stability of the enzyme during storage at 4°C and in the freezing/ thawing treatment. In all cases, the analysis of the microstructure of the beads was performed on the LNLS’ Small Angle X-ray Scattering SAXS2 beamline.
Source: Maria Victoria Traffano Schiffo, Tatiana Rocío Aguirre Calvo, Marta Castro-Giraldez, Pedro Jose Fito, and Patricio R. Santagapita, Alginate beads containing lactase: stability and microstructure. Biomacromolecules, DOI: 10.1021/acs.biomac.7b00202
Results are important for the production of fuel cells to direct ethanol
Findings can open the way to broad-spectrum antiviral drugs against ZIKV