
Pesquisador manipula célula eletroquímica usada no experimento
Experiment carried out on Sirius shed light on reaction fundamental to the production of hydrogen fuel
This text was originally published in Pesquisa FAPESP magazine in January 2023
A recent experiment at Sirius, the Brazilian synchrotron light source at the Brazilian Center for Research in Energy and Materials (CNPEM) in Campinas, São Paulo (see Pesquisa FAPESP issue 269) showed how a certain biological catalyst can more efficiently split water molecules (H2O) using electrolysis. This reaction, an electrochemical process that uses electricity to break down water into the elements that comprise it, is very significant because it produces not only oxygen but also hydrogen, considered the fuel of the future by many specialists because it does not emit any polluting gases when it is utilized (see Pesquisa FAPESP issue 314).
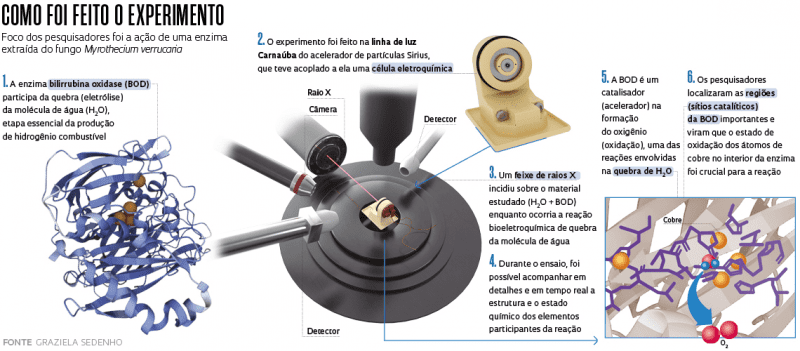
“We discovered that when some enzymes present in nature like bilirubin oxidase (BOD) are manipulated in the lab, they can accelerate the reaction to split water,” states chemist Frank Nelson Crespilho, a professor at the University of São Paulo’s São Carlos Institute of Chemistry (IQSC-USP) who led the study. “We didn’t know why this happened; thanks to new equipment developed specifically for Sirius, we were able to observe how this enzyme, BOD, behaves during the process of oxidation in water. We found that the copper atoms within it are relevant to this reaction.”
Crespilho expects this advance to pave the way for science to get inspiration from the part of the enzyme that accelerated the reaction. “It is important for us to recognize the important regions of BOD, since today synthetic chemists that work in materials production can copy and synthesize this part of the enzyme in the laboratory. This will make the catalyst much more affordable, with a much broader range of potential applications,” he adds. Most of the catalysts used in this process utilize noble metals like platinum and iridium, making large-scale application unfeasible due to the cost involved. An article describing the experiment written by Crespilho’s team, which includes the researchers Graziela Sedenho, Rafael Colombo, Thiago Bertaglia, and Jessica Pacheco, was published in October in the journal Advanced Energy Materials. Scientists from the Brazilian Synchrotron Light National Laboratory (LNLS) also participated in the study.
Researchers around the world look for new water oxidation catalysts
Bilirubin oxidase was extracted from the fungus Myrothecium verrucaria, which is frequently found in soil and plants. When manipulated in the lab, it participates in the water splitting reaction, which does not occur spontaneously in nature. Within the reactor, the enzyme works more specifically in the formation of molecular oxygen, one of the two reactions necessary to split the H2O molecule; the other reaction is hydrogen formation. Both occur at the same time. “To form hydrogen, which takes place on one side of the reactor, everything is well understood. There are cheap and efficient catalysts. But the other reaction, the oxidation of water, is very slow, and researchers around the world are searching for good catalysts for it,” explains Crespilho.
Extremely detailed observation of how the enzyme behaves during the bioelectrochemical reaction was only possible because of the infrastructure at Sirius. The testing utilized the Tarumã experimental beam station in the Carnaúba beamline, which is still in the process of scientific commissioning that involves testing and developing techniques, routines, and experimental strategies.
“Various types of scientific topics and experiments are addressed in this phase in order to show the beamline’s potential,” says physicist and researcher Helio Cesar Nogueira Tolentino, head of the Heterogeneous and Hierarchical Matter Division at the LNLS. Of the 14 lines initially planned for Sirius, seven are active. Each one operates a different beam using one main technique. The seven lines are open to scientists from Brazil and abroad.
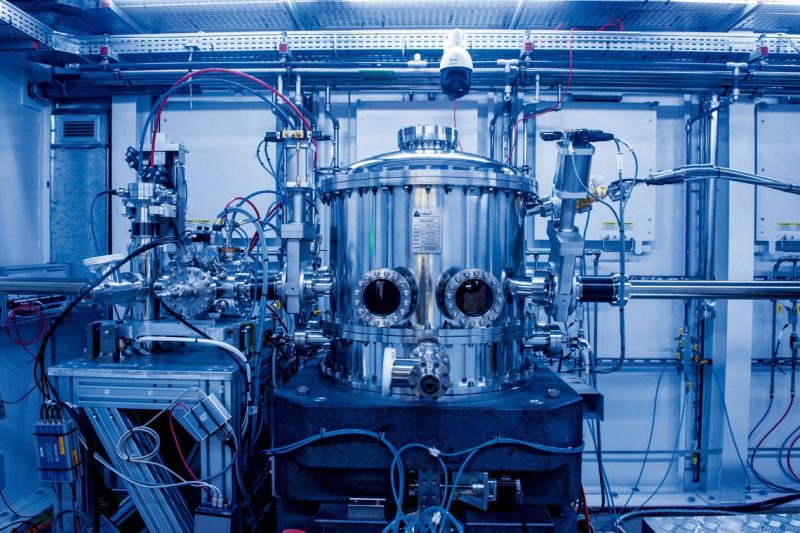
Monochromator: device is part of the Carnaúba beamline at Sirius, where the study was conducted (Léo Ramos Chaves/Pesquisa FAPESP
The Carnaúba beamline is the longest at Sirius, and has been operational since the second half of 2021. It was planned to conduct X-ray absorption spectroscopy, making it possible to conduct experiments on different materials on the nano scale. Besides a potent light line that produces a super-focused beam, Crespilho’s group was also able to use a new device developed by the LNLS team for the area of biochemistry.
“This is an electrochemical cell for in situ experiments. It is placed in front of the beam of X-rays that hit the material under study at the moment when a chemical reaction occurs. With this cell we can also apply electrical potential and measure or apply current and measure potential, in other words, we can see how the material responds to these external stimuli. All this while the chemical reaction is taking place,” says physicist Itamar Tomio Neckel, a researcher in the Carnaúba group at LNLS and the lead developer of the new electrochemical cell, a small device that fits in the palm of your hand.
The bigger challenge, he explains, is miniaturizing everything, since the reactions take place in an extremely limited physical space. At the same time, the conditions in different users’ laboratories must also be replicated. The Carnaúba beamline is 100 times finer than a human hair, according to the researchers, and becomes a X-ray nanoprobe.
Alexandre Affonso
The major difference is that this equipment makes it possible to map the experimental material in situ; in other words, researchers can visualize the state of the material (copper, in this case) during the different stages of the chemical reaction. “In in situ experiments, we study kinetics in real time. We generate an electrochemical reaction and study all the stages of the reaction using a microscope that provides information on the structure and chemical state of the elements within it, in real time,” explains Tolentino. “The experiments allow us to understand this process of bioelectrocatalysis, which is very important for producing hydrogen. They open the door to possibilities for producing hydrogen through a very simple reaction that uses common materials.”
Crespilho and his team’s efforts were part of roughly 30 projects from outside the LNLS accepted after a call for proposed experiments in October during the commissioning phase of the station. The article published by the group from IQSC-USP was the first in the area of bioelectrochemistry, but other experiments have already been performed with this beamline, including by a group from Argentina, and will soon be published.
“The results obtained by the group from USP in collaboration with the CNPEM show the potential for in situ electrochemical studies coupled with synchrotron radiation to shed light on the mechanisms of important reactions in biocatalysis,” says chemist Ana Flávia Nogueira of the Chemistry Department of Campinas State University (UNICAMP), who was not part of Frank Crespilho’s team. She stresses the groundbreaking use of this technique and its potential for research. “In this study, catalytic sites in copper can be identified to the nanometer. The partnership shows the Brazilian community how our researchers can benefit from the advanced techniques available at Sirius to gain importance and worldwide recognition in characterizing materials on the nano scale.”
The first scientific paper published with data obtained at the EMA beamline studied the relationship between skutterudite’s superconducting properties and the distance between their atoms.
Researchers from CNPEM and collaborators demonstrate the potential of naturally abundant and low-cost minerals for application in nanodevices