Research analyzes molybdenum catalysts doped with transition metals
The hydrogen gas ($\rm H_2$) is one of the best alternatives to fossil fuels because its combustion has as final product only water vapor. However, several technological challenges still need to be overcome in order to make it economically viable. One way to produce hydrogen is by breaking down water molecules $\rm H_2O$, with formation of $\rm H_2$ molecules. The main reaction in this process is the Hydrogen Evolution Reaction (HER) in which the protons in an acid medium are reduced and form hydrogen gas by electrons passed through catalysts.
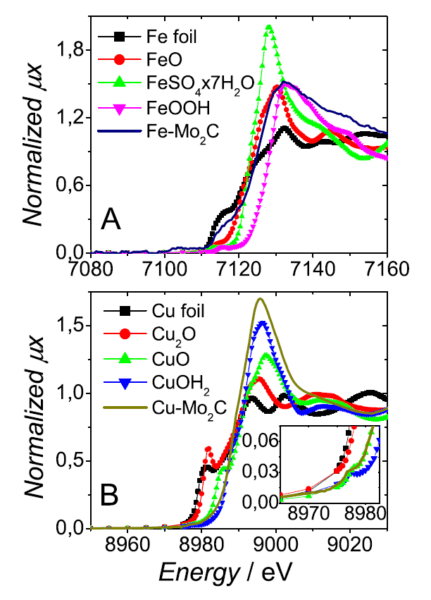
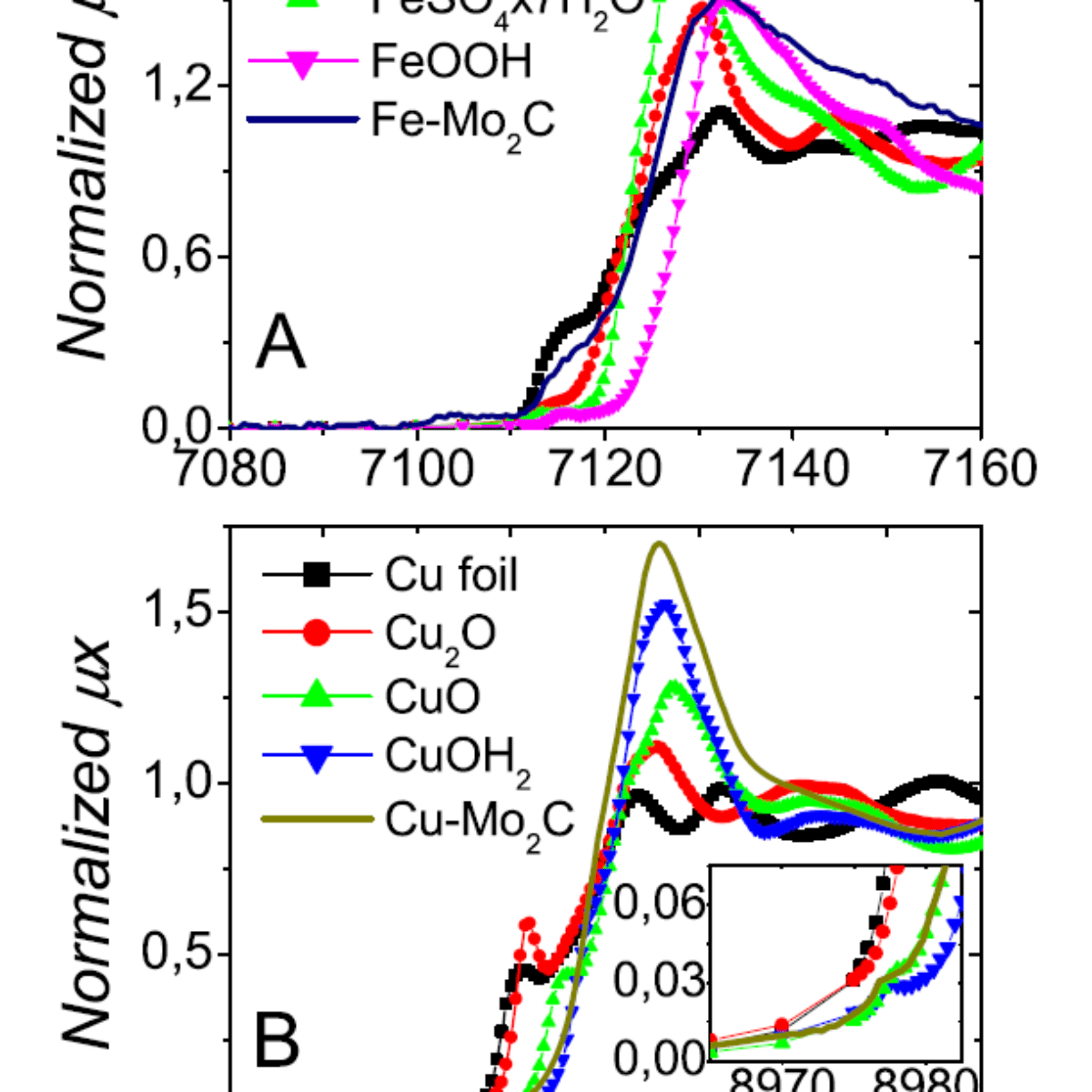
Figure 1: In situ XANES spectra of $\rm Fe-Mo_2C$ (A) and $\rm Cu-Mo_2C$ (B).
Among these catalysts, the most efficient is Platinum ($\rm Pt$), an expensive and scarce metal. The sustainable production of hydrogen requires cheaper catalysts, such as those based on molybdenum ($\rm Mo$). However, the activity of $\rm Mo$ catalysts for hydrogen production still needs to be improved.
Ana M. Gómez-Marín et al. [1] used the facilities of the Brazilian Synchrotron Light Laboratory (LNLS) to investigate the activity of Molybdenum Carbide ($\rm Mo_2C $) catalysts modified with transition metals (Iron, Cobalt, Nickel and Copper). The doping of catalysts with different chemical elements modifies the electronic and chemical properties of these materials, which can lead to the improvement of their activity.
The researchers evaluated the activity of the catalysts for the HER and their stability in acid media. Among the analyses, the group performed X-ray absorption measurements on the LNLS XAFS2 beamline to verify that the transition metals would not be removed from the catalysts in the electrochemical medium.
The results showed that doping with transition metals increases the stability of the catalysts. However, the doping slightly reduced the activity of the catalysts when compared to the non-doped Molybdenum Carbide, in the order: $\rm \alpha-Mo_2C $> $\rm Fe-Mo_2C $> $\rm Co-Mo_2C $> $\rm Ni-Mo_2C $> $ \rm Cu-Mo_2C $.
Nevertheless, according to the group, a high activity in the HER reaction was measured for all the $\rm TM-Mo_2C$ catalysts, which demonstrates their potential for use in the production of hydrogen.
Source: [1] Ana M. Gómez–Marín, Edson A. Ticianelli, Effect of transition metals in the hydrogen evolution electrocatalytic activity of molybdenum carbide, Applied Catalysis B: Environmental, 209, 2017, pp 600-610. DOI: 10.1016/j.apcatb.2017.03.044.
Research uncovers the mechanism of memory effect of lamellar double hydroxides (LDH).
Research analyzes the effect of nanoparticles formed during the preparation of Li-RHC with addition of $\rm TiO_2$