
Research develops nanostructured material with high oxygen storage and release capacity for the improvement of catalytic converters
Complete combustion of both fossil and biofuels generates carbon dioxide ($\rm CO_2$) and water as final products. However, incomplete combustion of these substances can occur in automobile engines, generating important pollutants such as carbon monoxide ($\rm CO$), hydrocarbons, and nitrogen oxides (such as $\rm NO$ and $ \rm NO_2$).
To reduce the emission of these toxic substances, an equipment called a catalytic converter is used in the exhaust of vehicles. Materials called catalysts promote and accelerate chemical reactions without being consumed during the process. They retain on their surface the reactant molecules, weakening the bonds between the atoms and causing the pollutants to be converted into less harmful gases.
The action of the catalytic converter happens in three stages. The first stage converts the nitrogen oxides into nitrogen ($ \rm N_2$) and oxygen ($ \rm O_2$) gases. The second stage breaks down bonds of unburnt hydrocarbons and carbon monoxide, turning them into $ \rm CO_2$. Finally, the third stage has an oxygen sensor to regulate the intake of air and fuel to the engine, so that the amount of oxygen is always close to the most efficient for the different reactions.
The efficiency of the oxidation and reduction reactions depends on the amount of oxygen available to the catalyst. Therefore, catalytic converters benefit from the inclusion of materials that have high capacity to store and release oxygen under oxidation and reduction situations, respectively. This property is called Oxygen Storage Capacity (OSC). The OSC mechanism depends significantly on the amount of oxygen in the local atmosphere. Therefore, the materials respond rapidly to the variation of oxygen in the exhaust and allow the catalyst to maintain on its surface the proper ratio between the reactants, whatever the regime of the engine.
Materials based on cerium ($\rm Ce$) present great potential for catalytic applications, both as a catalyst and to improve the performance of other materials. Cerium oxide (IV) ($\rm CeO_2$), also called ceria, has a high OSC. As a result, car manufacturers have systematically incorporated cerium-doped oxides into the formulations of catalytic converters used to control gas emissions.
By controlling the shape and surface of ceria nanoparticles, it is possible to optimize the OSC property. However, the tendency of nanoparticles to coalesce into larger crystals limits their use for impregnation of catalysts without the addition of other materials. To avoid it, one possibility is the dispersion and confinement of the cerium oxide nanoparticles in the pores of a mesoporous ordered structure.
In this context, Juliana Fonseca et al. [1] synthesized a series of samples of $\rm CeO_2$ deposited on orderly mesoporous alumina (OMA) with different proportions of $\rm Ce$, material denoted as $\rm CeXAl$. The samples, with very small and well dispersed ceria nanometric clusters, were characterized in depth with attention to their intrinsic redox properties.
Among the several analyses carried out by the group, the XAFS2 beamline from the Brazilian Synchrotron Light Laboratory (LNLS) was used to monitor the changes in structure and oxidation state of the catalysts using X-ray absorption spectroscopy (XANES) in situ.
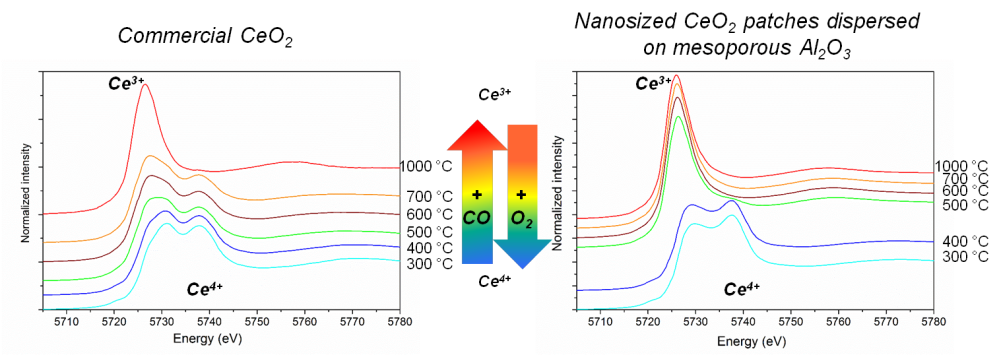
The simulation of redox processes on the $\rm CeXAl$, by the alternate introduction of atmospheres of hydrogen ($\rm H_2$) and oxygen, simultaneously with the XANES measurements, showed the complete and reversible reduction of Ce (IV) into Ce (III) between 400 and 500 °C.
The complete experimental data allowed the researchers to conclude that the reduction of $ \rm Ce$ was associated to the reaction between the ceria and alumina surface, following the equation $ \rm 2CeO_2 + Al_2O_3. + H_2\, (or\, CO) \rightarrow 2CeAlO_3 + H_2O\, (or\, CO_2)$. When the reduction temperature did not reach the crystallization temperature of the $\rm CeAlO_3$, the regeneration in $\rm CeO_2$ and $\rm Al_2O_3$ was complete and rapid at 400 ° C.
The redox properties were also investigated in comparison to commercially available ceria. The group showed that, in the $\rm CeXAl$ samples, Ce (IV) was completely reduced into Ce (III) between 400 and 500°C, while less than 10% of the Ce (IV) in the commercial sample was reduced at the same temperature.
The researchers conclude that such nanostructured material exhibiting high OSC may have numerous applications in heterogeneous catalysis for various oxidation reactions, or for systems where good ionic conductivity is required.
Source: [1] Juliana Fonseca, Nicolas Bion, Yordy E. Licea, Cláudia M. Morais, Maria do Carmo Rangel, Daniel Duprez and Florence Epron. Unexpected redox behaviour of large surface alumina containing highly dispersed ceria nanoclusters. Nanoscale (2019) 11, p.1273. DOI:10.1039/C8NR07898J
Results contribute to improve processing technology and valorization of this resource
Research investigates the chemical nanostructure of water conducting vessels