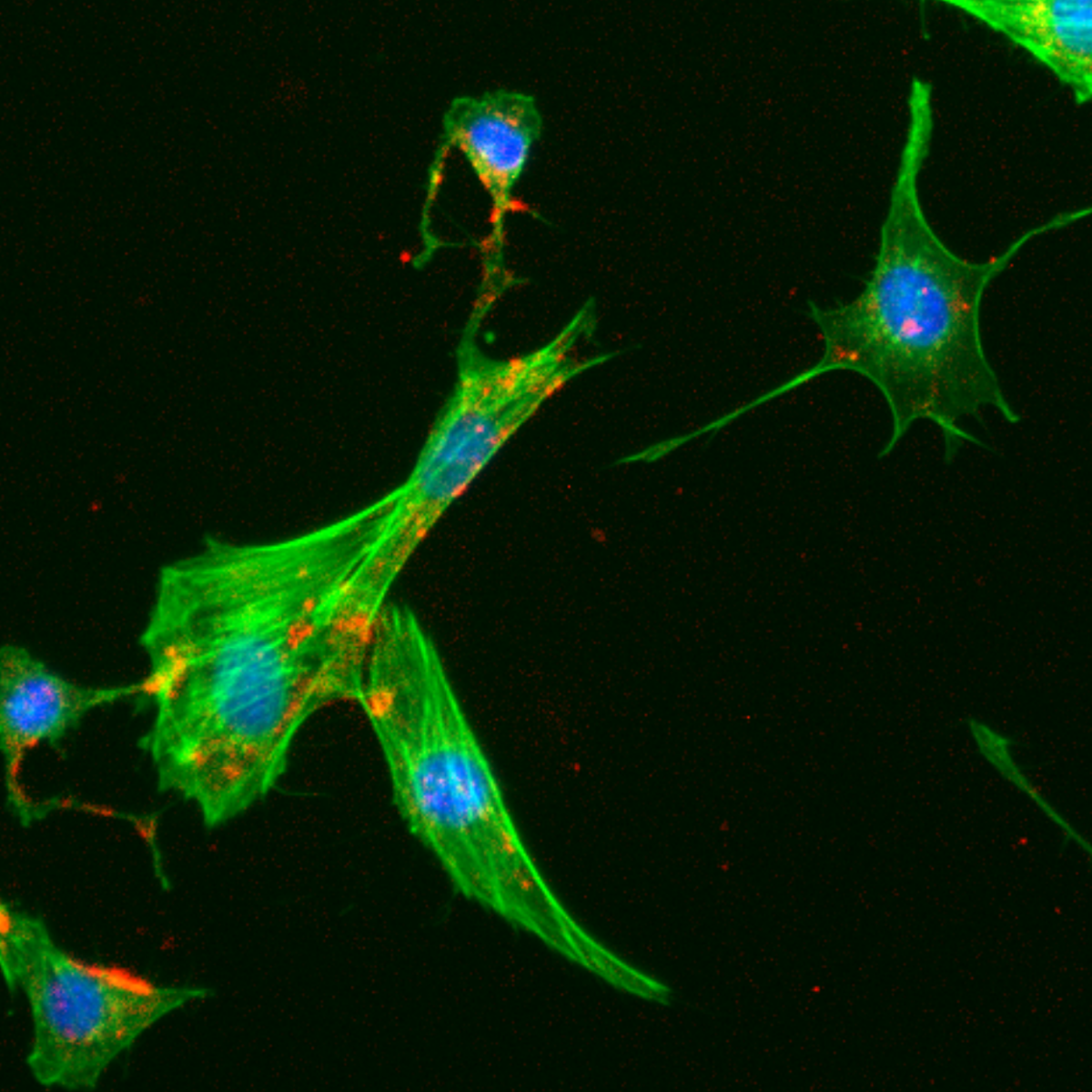
Fibroblasts after incubation with silica nanoparticles in the presence of bovine serum albumin (BSA). Wide-field fluorescence microscopy image. (Image adapted from Galdino et al., Small, 2024, https://doi.org/10.1002/smll.202409065)
Research led by CNPEM scientists reveals intracellular movement of nanoparticles coated with a protein corona. The technique employed in the study will be available at the Sibipiruna beamline, which is dedicated to research on BSL-4 pathogens.
Researchers from the Brazilian Center for Research in Energy and Materials (CNPEM), in collaboration with institutions in Brazil, the United Kingdom and the United States, have demonstrated a new strategy to monitor the intracellular trajectory of nanoparticles. The study was one of the cover features for the journal Small in June 2025, and combined different high-resolution microscopy techniques to observe how these particles move around in the cellular environment over time.
The research used advanced microscopy resources from the Nanotechnology National Laboratory (LNNano) and Sirius facilities, and included a technique not yet available at the center, X-ray cryotomography. Measurements were obtained using a beamline with characteristics similar to the future Sibipiruna line, which will be part of Project Orion. The resulting data verified the use of this technique in cryogenic conditions, as well as its ability to reveal cellular structures smaller than the viruses that will be studied in the future laboratory complex.
The approach made it possible to identify the migration of nanoparticles to the perinuclear region of cells and fusion of the vesicles that transport them, without the use of contrast agents. The results overcome common limitations in studies of this type, and offer a promising tool for understanding how nanomaterials behave in complex biological systems.
Nanoparticles have been widely studied for their potential in biomedical applications such as controlled release of medications, diagnostic imaging and targeted therapy. But these applications still face major obstacles, especially with regard to detailed understanding of the mechanisms through which these particles are internalized and move within cells.
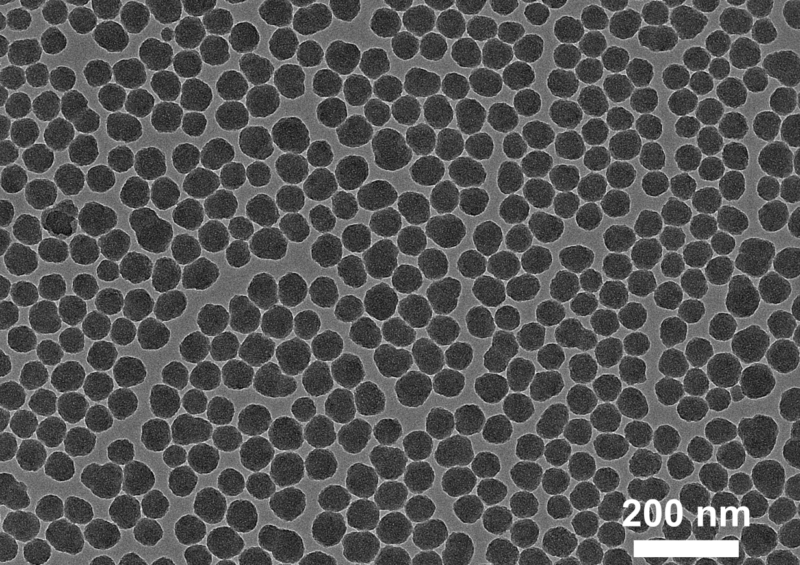
Non-functionalized silica nanoparticles observed under transmission electron microscopy (TEM). (Image adapted from Galdino et al., Small, 2024, https://doi.org/10.1002/smll.202409065)
The formation of the protein corona, a layer of biomolecules that adsorbs onto the surface of nanoparticles when they come into contact with biological fluids, is a good example of the complexity involved in investigating the mechanisms of internalization and intracellular transit. This layer significantly alters the physical and chemical properties of the particles, influencing their stability and interaction with different cell types. Understanding the behavior of these nanoparticles in the intracellular environment consequently requires approaches that take into account both cell dynamics and the variability introduced by the corona’s composition.
Despite advances in characterization techniques, most studies offer only specific or static views of the internalization process; it is generally not possible to distinguish between particles absorbed at different times, or to follow their precise location within the cell over time. This study conducted by CNPEM researchers proposes an alternative approach intended to overcome these obstacles through an experimental strategy that employs different imaging methods, providing a broader analysis that extracts the best from each technique.
The researchers proposed a protocol based on a short period of cell exposure, followed by complete removal of the unabsorbed nanoparticles and cryopreservation of the cells after different time intervals (0, 2, and 24 hours). Nanoparticle internalization by the cells was then evaluated using wide-field fluorescence microscopy, and showed progressive migration to the perinuclear region.
“Previous studies investigating the internalization process of nanoparticles also used cells that were fixed after different time intervals but incubated continuously. As a result of this method, nanoparticles internalized at the beginning of the incubation period cannot be distinguished from those internalized at the end. The alternative method we are proposing avoids this problem and facilitates analysis of the sequence of events and changes associated with the cell internalization process,” explains Mateus Cardoso, an author of the article and researcher at the Synchrotron Light National Laboratory (LNLS), which is part of CNPEM.
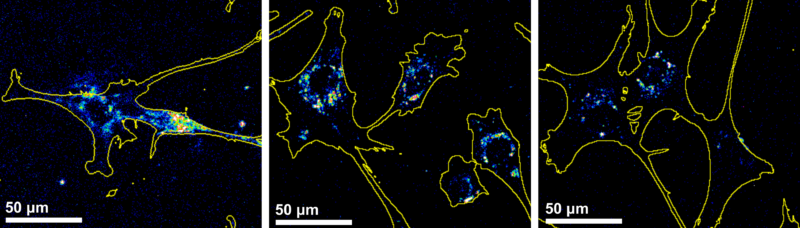
Images obtained by wide-field fluorescence microscopy at 0h, 2h and 24h after incubation. (Image adapted from Galdino et al., Small, 2024, https://doi.org/10.1002/smll.202409065)
To better understand the intracellular trajectory of the nanoparticles, the researchers used transmission electron microscopy (TEM) images obtained at the Nanotechnology National Laboratory (LNNano) at CNPEM. This technique offers high resolution and the ability to visualize subcellular structures in great detail. The images revealed that the nanoparticles always remain inside vesicles and are not released into the cytosol. It was also possible to observe different stages of the route taken by these vesicles (including signs of fusion between compartments), which helps researchers understand how cells process nanoparticles over time.
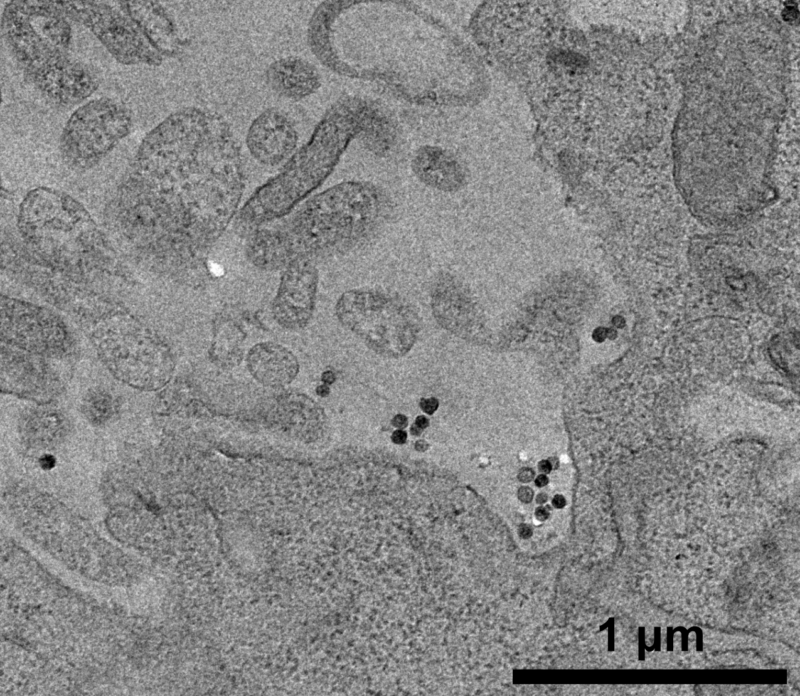
Transmission electron microscopy (TEM) image showing silica nanoparticles (SiNP) being internalized by cells through the formation of vesicles. (Image adapted from Galdino et al., Small, 2024, https://doi.org/10.1002/smll.202409065)
Although it provides high-resolution images, transmission electron microscopy (TEM) has important limitations such as low electron penetration, which requires physical cutting of samples and prevents three-dimensional visualization. Additionally, the fixation and dehydration processes required for preparation can alter the morphology and composition of cell structures.
In contrast, cryo-soft X-ray tomography (cryo-SXT) allows observation of whole, hydrated cells in 3D without the need for ultra-thin sections or stains, contributing to a more faithful visualization of the intracellular organization and environment. The images obtained by the researchers using this technique revealed that the silica nanoparticles remain confined in vesicles, which expand over time, suggesting that they undergo processes of fusion and reorganization.
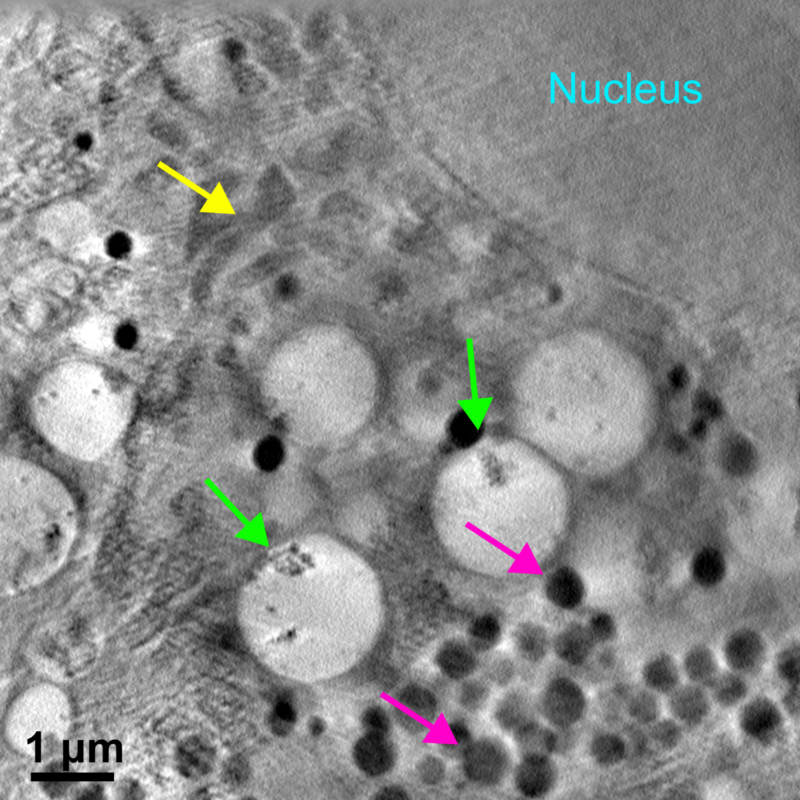
Image of section obtained via cryotomography 24 hours after removal of the nanoparticles that were not absorbed. Green arrows indicate vesicles containing silica nanoparticles. (Image adapted from Galdino et al., Small, 2024, https://doi.org/10.1002/smll.202409065)
Along with these approaches, the researchers also used cryogenic structured illumination microscopy (cryoSIM), which makes it possible to identify specific cellular components with high spatial precision. By combining this technique with pH-sensitive fluorescent markers, they were able to detect acid vesicles (such as late endosomes and lysosomes) and correlate them with the presence of the nanoparticles. The cryo-SXT and cryoSIM analyses were carried out at the British synchrotron Diamond Light Source; its advanced infrastructure was essential for generating high-resolution three-dimensional images and accurately mapping the intracellular compartments.
“By combining complementary imaging techniques, we can analyze how these nanoparticles behave over time in much more detail. Cryo-SXT, for example, confirmed the increase in vesicle size and the number of nanoparticles per vesicle, revealing important clues about the internal reorganization of these compartments. Meanwhile, super-resolution 3D microscopy showed that the nanoparticles can remain in cellular environments with different levels of acidity, which indicates they persist in multiple intracellular contexts,” adds Cardoso.
In the future, Brazil will also have a dedicated infrastructure for this type of research. The Sibipiruna beamline, which is being developed at Sirius as part of Project Orion, will specialize in soft X-ray computed tomography. Its instrumentation will allow users to obtain quantitative three-dimensional maps with nanometric resolution of structures and organelles in cells infected by high-risk pathogens, an important advance for studies in complex cellular environments.

Plan for Orion (Photo credit: Outreach/CNPEM)
As Mateus Cardoso states, “This study has already benefited from the current capacities of the open facilities available at CNPEM. As beamlines like Sibipiruna come online in the future, we will be able to apply advanced soft X-ray tomography techniques in research involving high-risk pathogens.”
The discoveries made during this research broaden understanding of the behavior of nanoparticles inside cells. The combination of imaging techniques at different scales, from electron microscopy to cryotomography, demonstrates the potential of multimodal approaches to answer complex questions in modern cell biology. This strategy can be applied to a wide variety of systems, contributing to research in areas like drug delivery, diagnostic imaging and research on infectious and degenerative diseases.
“This is the first time we’ve put together a task force to use a beamline that involves a technique that is not yet available at CNPEM but will be a flagship program at Orion. The beamline is very similar to what Sibipiruna will offer. And with this we were able to prove that it is possible to take measurements in cryogenic conditions and obtain data about structures even smaller than the viruses that will be studied at Orion,” concludes Cardoso.
The Brazilian Center for Research in Energy and Materials (CNPEM) is a state-of-the-art, multi-user and multidisciplinary scientific environment with activities on different fronts within the Brazilian National System for Science, Technology and Innovation. A social organization overseen by the Ministry of Science, Technology and Innovation (MCTI), CNPEM is driven by research that impacts the areas of health, energy, renewable materials, and sustainability and is responsible for Sirius, the country’s largest scientific research infrastructure. CNPEM is currently constructing Project Orion, a laboratory complex for advanced pathogen research. Highly specialized science and engineering teams, sophisticated infrastructure open to the scientific community, strategic lines of investigation, innovative projects involving the productive sector, and training for researchers and students are the pillars of this institution that is unique in Brazil and able to serve as a bridge between knowledge and innovation. Research and development activities at CNPEM are conducted by its National Laboratories in the areas of Synchrotron Light (LNLS), Biosciences (LNBio), Nanotechnology (LNNano), and Biorenewables (LNBR), as well as the Ilum School of Science, which offers a bachelor’s degree program in science and technology with support from the Ministry of Education (MEC). https://cnpem.br/
Scientists investigated the magnetic properties of overlapping layers of platinum, cobalt and gadolinium
Study suggests eucalyptus could be the key to a sustainable and cost-effective solution for recovering manganese-contaminated soils