Reaction is an important step in the transformation of carbon dioxide into fuels
In 2016, the concentration of carbon dioxide ($\rm CO_2$) in the atmosphere has exceeded 400 ppm (parts per million), and has increased year by year since the beginning of the industrial revolution, when human activity began to inject more carbon into the atmosphere than the what the environment is capable of absorbing. The increase in the concentration of both $\rm CO_2$ and other greenhouse gases in the atmosphere is currently considered the main cause of the rise in the average temperature of the planet, which leads to the intensification of extreme climatic events.
Because of this, demand for both alternative energy sources that reduce fossil fuel consumption and methods to reduce the impact of carbon dioxide overproduction has intensified in recent years. An example is the transformation of $\rm CO_2 $ into hydrocarbons for the production of fuels and raw materials for the chemical industry.
There are several ways to convert $\rm CO_2$ into hydrocarbons, some of which require as an intermediate step the conversion of $\rm CO_2$ into carbon monoxide ($\rm CO$). , The reaction $\rm CO_2 + H_2 \rightleftharpoons CO + H_2O$, called Reverse Water Gas Shift (RWGS), is one of the main ways to accomplish this conversion. Subsequently, $\rm CO$ produced in this controlled manner can be converted into hydrocarbons by the so-called Fischer-Tropsch synthesis.
However, the efficient conversion of $\rm CO_2$ into $\rm CO$ depends on the use of catalysts that make the process more selective, i.e., avoid significant production of products other than carbon monoxide. One of those unwanted products is methane ($\rm CH_4 $), which is produced in the methanation reaction that may occur concomitant to the RWGS reaction.
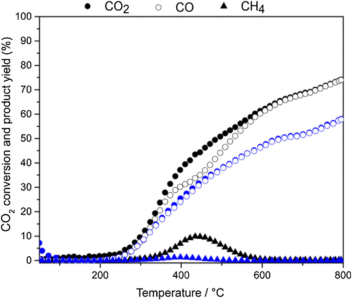
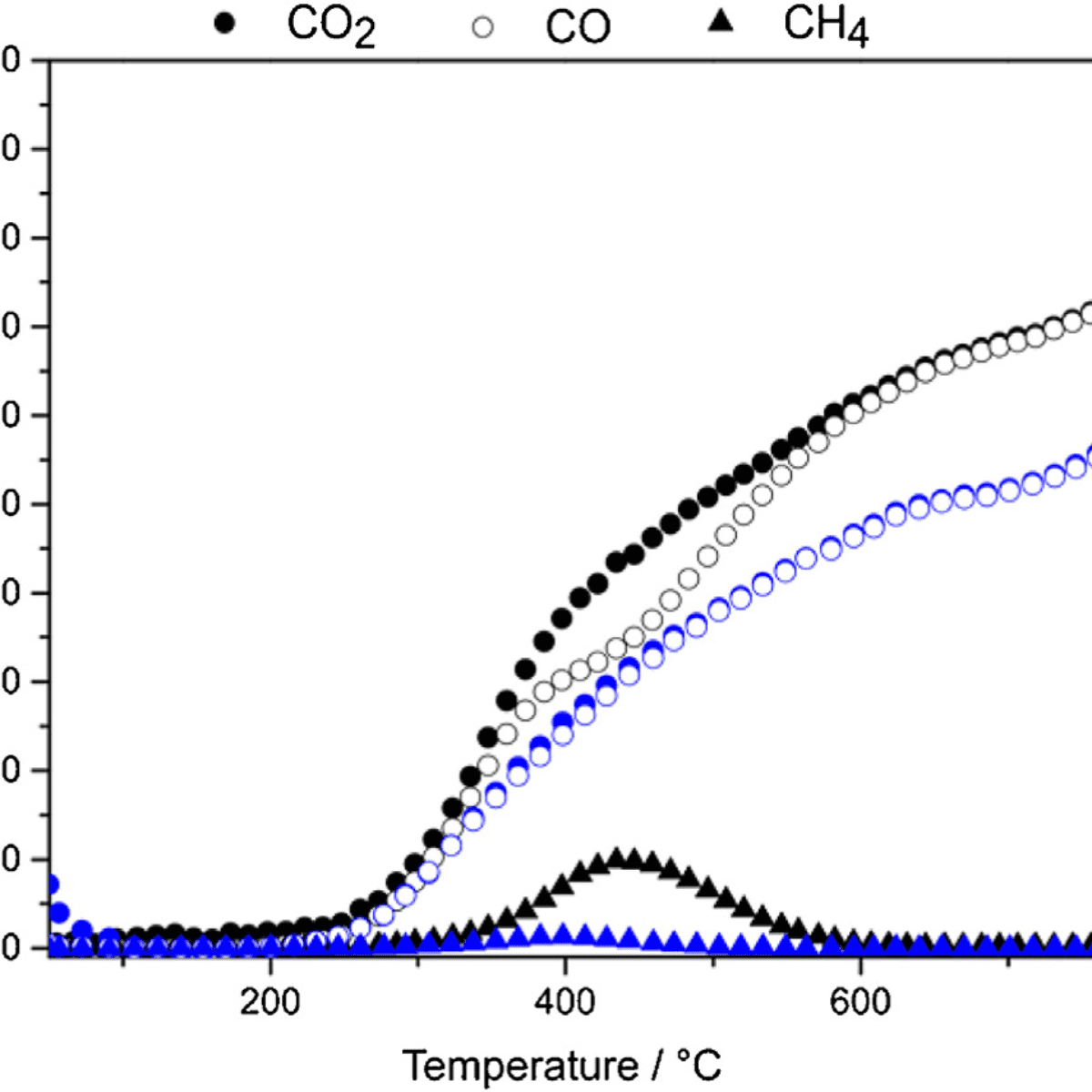
Figure 1: $\rm CO_2$ conversion and $\rm CO$ and $\rm CH_4$ formation on $\rm Ni/nSiO_2$ catalyst as a function of temperature using different $\rm CO_2:H_2$ ratio: 1:4 (black) and 1:1 (blue).
A group of researchers from the University of São Paulo, University of Lille (Lille, France) and Brazilian Synchrotron Light Laboratory (LNLS) and Brazilian National Nanotechnology Laboratory (LNNano) used the LNLS facilities to analyze nanoparticulate nickel $\rm Ni$ catalysts to perform the conversion of $\rm CO_2$ to $\rm CO$.
The researchers used silica nanospheres ($\rm nSiO_2$) as support for the catalyst. The small size of the support increases the surface area available for deposition of $\rm Ni $. The deposition was done by the magnetron sputtering technique, which allows the control of the size and composition of the metal particles more efficiently than typical wet-impregnation methods of deposition.
The $\rm Ni/nSiO_2$ catalyst was used in the RWGS reaction with different proportions between the $\rm CO_2$ and the $\rm H_2$ gases. The production of $\rm CO$ was predominant throughout the studied temperature range, with very low methane production. Above 600°C, $\rm CO$ was the only product observed with conversions close to the thermodynamic equilibrium. In the stability studies, the catalyst showed a conversion rate of 64% with 100% selectivity for $\rm CO$ for 40 hours and still showed stable activity between two thermal cycles.
The LNLS Small Angle X-ray Scattering SAXS1 Beamline was used the analysis of the size of the silica nanospheres and the XAFS1 Absorption Spectroscopy and X-Ray Fluorescence Beamline was employed to analyze the active phase of the catalyst, both as prepared and at different temperatures under the conditions of operation of the RWGS reaction.
The high conversion and selectivity for the RWGS reaction and long-term stability presented by the catalyst developed by the group is a successful step in the conversion of $\rm CO_2$ to $\rm CO$ for the subsequent hydrocarbon production.
Source: Renato V. Gonçalves, Lucas L.R. Vono, Robert Wojcieszak, Carlos S.B. Dias, Heberton Wender, Erico Teixeira-Neto, Liane M. Rossi, Selective hydrogenation of $\rm CO_2$ into $\rm CO$ on a highly dispersed nickel catalyst obtained by magnetron sputtering deposition: A step towards liquid fuels, Applied Catalysis B: Environmental, v. 209, p. 240-246. DOI: 10.1016/j.apcatb.2017.02.081.
Study shows the validity of mini-brains as a model for embryonic neurodevelopment.
A detailed characterization of platinum-cerium-alumina model catalysts under Water Gas Shift conditions have been performed.