Our brain gives movement, shape and color to the world through the light that is produced by the Sun, by lamps or flames, which is reflected by the objects around us and captured by our eyes. However, the colors we see are not an intrinsic property of the light that reaches our eyes. They are, in fact, the interpretation that our brain gives to the electrical signal produced in the retina in response to the incidence of light.
The retinal cells are sensitive to only a tiny fraction of all types of light that exist, called electromagnetic waves. In addition to the light that is visible to us and that provides us with a lot of information about the world around us, there are still so many electromagnetic waves, so many other types of light, that we cannot see.
This is the case, for example, with ultraviolet light produced by the sun. Although we cannot see it, it can cause damage to our eyes, in addition to being one of the factors responsible for the occurrence of skin cancer. On the other hand, bees and butterflies have eyes capable of detecting ultraviolet radiation and are guided by it in search of food, as they see colors in flowers that we cannot see.

Under ultraviolet light, the flowers of some plants, such as sunflowers, have a darker center and also specific patterns on the petals. These patterns are visible to pollinators, who are guided to where the nectar is located.
The study of these invisible types of light allowed the development of the most diverse technologies, such as the radio waves transmitted by radio devices and Wi-Fi networks, microwaves in microwave ovens and cellphone networks, infrared light from remote controls and automatic doors, ultraviolet radiation used in the sterilization of objects and in the treatment of water, X-rays in hospital radiographs and tomography, and the gamma rays used in cancer therapies.
These technologies are the result of the advancement of scientific knowledge, which is closely linked to the development of tools that allow us to visualize and understand the structure of matter on scales that normally our eyes cannot see.
For example, for us to know that living beings are composed of cells, it was necessary the invention and improvement of the optical microscope, between the end of the 15th century and throughout the 16th century. In this equipment, light is reflected by the material and pass through a set of lenses that amplify the image so that our eyes can perceive details that were previously inaccessible. Later, this knowledge served as a basis for us to understand that many diseases are caused by microorganisms invisible to the naked eye.
Our ability to observe and understand the world around us advances as new tools become available.
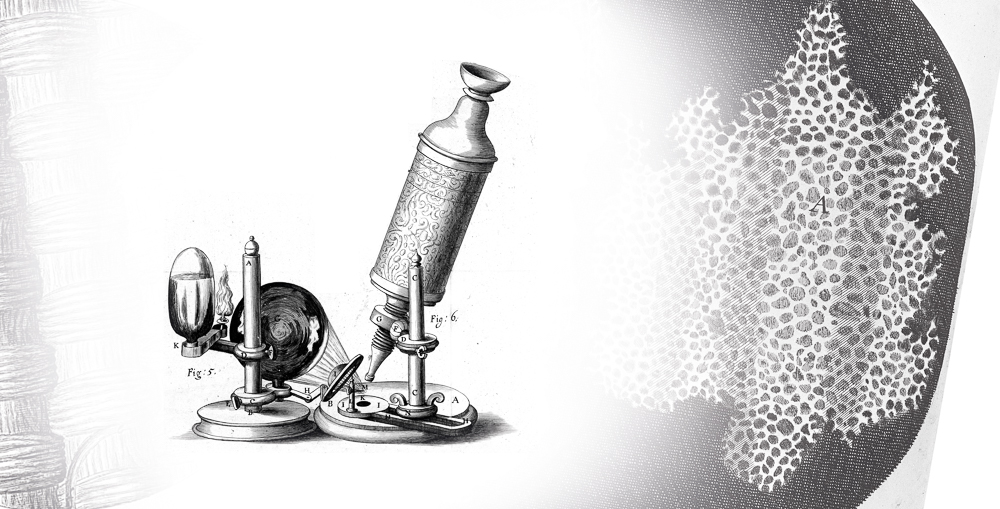
Microscope used by Robert Hooke to study cork cuts. Hooke describes what he called “cells” (from the Latin “cella”, a small cavity). This observation gives rise to the word used today to describe the smallest unit of a living organism. However, the structures observed by Hooke are actually the walls of dead plant cells.
Today, it is also known that all things, living or not, are made up of atoms. These atoms are composed of a positively charged nucleus and negatively charged electrons that orbit the nucleus in a stable manner. Each material is composed of different types of atoms, that is, different chemical elements, which bond in different ways.
The way in which the atoms of a substance are distributed in space will then define the distribution of electrons throughout the material – its electronic structure. The macroscopic properties of a material depend on this structure – whether it will be rigid or malleable, opaque or transparent, conductive or insulating. Understanding the properties of materials requires, therefore, that we know the atoms that compose them and how they are distributed.

Diamond and graphite, two materials with completely different properties, are composed of the same carbon atoms, the only difference being their spatial arrangement.
The way in which electromagnetic waves interact with materials – whether they are reflected or absorbed by objects – depends, among other factors, on the atoms that constitute them. Not only that, but each chemical element has a kind of fingerprint that allows it to be identified by the way it interacts with electromagnetic waves.
Thus, in the same way that visible light allows us to observe macroscopic characteristics of things, it is possible to use both visible light and all other diverse electromagnetic waves to investigate the structure, composition and properties of things on the microscopic scale. They allow us to open a new window for observing the world around us.

When sunlight passes through an equipment similar to a prism, it is possible to observe all the different types of visible light emitted by the Star. The dark bands correspond to the light absorbed by the gases on the surface of the Sun. Each chemical element absorbs very specific bands of light and, therefore, from the analysis of that missing light, it is possible to identify which are the chemical elements present on the surface of the Sun. For example, from such an analysis the chemical element Helium was first discovered on the Sun in 1870, years before the discovery of its presence on Earth.
Image: N.A.Sharp, NOAO/NSO/Kitt Peak FTS/AURA/NSF
Many of the challenges that Brazil and the world face today require increasingly sophisticated scientific and technological developments. Whether creating drugs against new diseases, using biomass as a source of renewable energy, or designing new fertilizers for more sustainable agriculture, understanding and manipulating the microscopic mechanisms behind macroscopic processes is essential.
For this, advanced tools are needed, which allow investigating all types of materials, whether biological or synthetic, with high spatial resolution for viewing micro and nanometric details. It is also important to investigate materials in different size scales and with high temporal resolution, for the study of phenomena that occur in fractions of seconds. Finally, all of this must be investigated under the real conditions in which the material will be used, if possible with the detailing of different information, such as the concentration and distribution of its chemical elements and their chemical bonds.

Synchrotron light is a type of extremely bright electromagnetic radiation that extends over a wide spectrum, that is, it is composed of several types of light, from infrared, through visible light and ultraviolet radiation, and reaching X-rays.
With the use of this special light it is possible to penetrate matter and reveal characteristics of its molecular and atomic structure for the investigation of all types of material. Its wide spectrum allows carrying out different types of analysis using each of different kinds of light of which synchrotron light is composed. Its high brightness allows extremely fast experiments and the investigation of details of materials at the nanometer scale. With synchrotron light it is also possible to follow the evolution in time of physical, chemical, and biological processes that occur in fractions of a second.
The characteristics of this light also allow these analyzes to be made while the materials are submitted to different conditions of temperature and pressure, of vacuum and flow of different gases, of electric and magnetic fields, and many other variables. Thus, it is possible to carry out experiments under the same conditions in which the samples are found in nature – such as inside the earth’s crust – or under the conditions in which the materials will be used, as in industrial processes, for example.