CONTACT & STAFF
For more information on this beamline, contact us.
The DXAS beamline is an experimental station dedicated to dispersive x-ray absorption spectroscopy (acronym for DXAS) techniques, in the hard x-ray energy range (5 to 14 keV). The peculiarity of this beamline is the capability to collect absorption spectra over an extended range of photon energies without any mechanical movement of its optical elements. The DXAS is especially suited for detecting weak signals in XANES (X-ray Absorption Near-Edge Spectroscopy) and XMCD (X-ray Magnetic Circular Dichroism) experiments and for tracking time-dependent evolution of chemical reactions.
DXAS is installed on a 1.67T bending-magnet source, and it was opened to users in 2005. The beamline is comprised by the synchrotron light source, a vertically focusing bendable mirror, a bent crystal polychromator, and an area detector. The beam path over the optical elements starts when it hits the bendable mirror, used for vertical focusing as well as harmonic rejection. Then the light beam impinges onto a polychromator bent crystal at several different incident angles, resulting in a polychromatic beam after reflection. The reflected beam is selected with a specific bandwidth of hundreds of eV, and is horizontally focused at the sample position. The transmitted signal, after the sample position, reaches an area detector. The photon energy–direction correlation is transformed into an energy–position correlation along the horizontal axis of the detector.
The main features of the beam line are fast acquisition and stability. A whole X-ray absorption spectrum is acquired in a single detector shot. Thus, it makes the technique especially useful for the study of fast processes. Due to the absence of movement of the optical elements during the data acquisition, the focused beam at the sample position is inherently stable.
The beamline has been used to support studies in the fields of materials science, solution chemistry, heterogeneous and homogeneous catalysis, electrochemistry, magnetism and geosciences.
For more information on this beamline, contact us.
The following experimental techniques and setups are available to users in this beamline. To learn more about the techniques’ limitations and requirements (sample, environment, etc.) contact the beamline coordinator before submitting your proposal.
X-ray absorption spectroscopy (XAS) is a widely used technique for determining the local geometric on the atomic scale and/or electronic structure of matter. It provides information about a selected element in a material and is very effective for establishing structure-property relationships in any kind of materials.
Setup: Time-resolved XAS measurements in transmission mode
This setup is optimised for time-resolved measurements in transmission mode of homogeneous samples. The high penetration of hard X-rays allows working with a wide variety of sample environments (with gas, liquid, pressure…) in order to perform in situ and even operando time-resolved experiments that are crucial to better understand materials. Only transmission measurements are available on the DXAS beamline and the sample environment must fit within this restriction. Depending of the sample and sample environments, milliseconds time resolution is available.
X-ray magnetic circular dichroism (XMCD) technique is used to determine the element, orbital and spin magnetic properties of a material as a function of the environmental condition of the sample (temperature, pressure, applied field).
Setup: XMCD in transmission mode
This setup is optimised for transmission detection of XMCD signal, where the sample is between the pole pieces of a electromagnet with field up to 1 T and inside a cryostat allowing temperatures in the range of 20 to 300 K. High pressure anvil cell can also be employed for pressures up to 50 GPa.
Setup: XMCD in reflectivity mode
This setup is optimised for detection of the dichroic signal at the reflectivity of the x-rays from thin film samples, where the sample is between the pole pieces of an electromagnet with field up to 1 T.
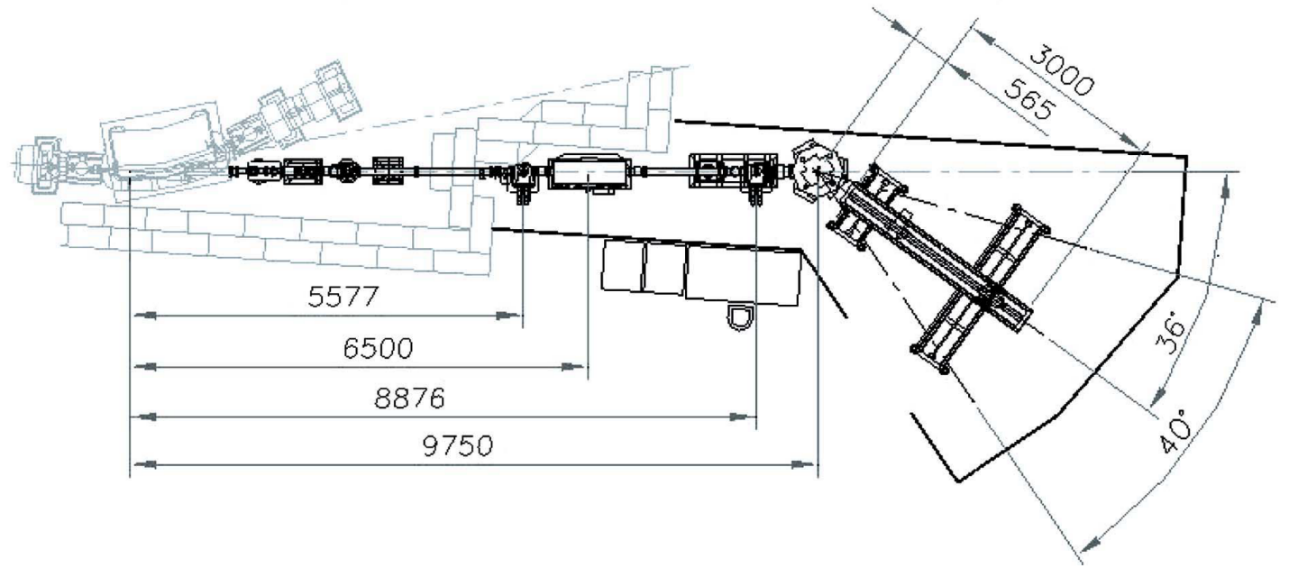
| Element | Type | Position [m] | Description |
|---|---|---|---|
| Source | Bending magnet | 0.00 | Bending Magnet D06 exit A (4°), 1.67 T, 750 µm x 168 µm |
| Mirror | Vertical focusing mirror | 6.50 | 800 mm long Rh coated |
| Crystal | Crystal polychromator | 9.75 | Water-cooled Si(111) |
| Parameter | Value | Condition |
|---|---|---|
| Energy range [keV] | 5 – 14 | Si(111) |
| Energy resolution [ΔE/E] | 13.1 x 10-5 | Si(111) |
| Energy band-pass [eV] | Hundreds of eV | – |
| Beam size at sample [µm2, FWHM] | 150 x 200 | at 8 keV |
| Photon flux at sample [ph/s] | 2 x 1011 | at 8 keV |
| Instrument | Type | Model | Manufacturer | Specifications |
|---|---|---|---|---|
| Detector | Area detector | Pylon2048F | – | Princeton Instruments |
| Furnace | Capillary, provide attachments to gas lines | – | Max. temp.: 1000°C Max. heating ramp: 20°C/min Quartz capillary inner/outer diameters [mm]: 0.8/1.0; 1.0/1.2 and 2.0/2.4 | LNLS in-house development |
| Furnace | Tubular, provide attachments to gas lines | – | Max. temp.: 1000°C Max. heating ramp: 20°C/min Pellet sample with a diameter of 13 or 6 mm | LNLS in-house development |
| Mass flow controllers | – | – | Gas flow [mL.min-1]: 0.2 – 750 | BROOKS |
| Gas cylinders | – | – | Pure gases: Ar, He, N2, synthetic air Gas mixture (% diluted in He): CO (20%), O2 (5 and 40%), H2 (5%), CO (5%), NO (5%), CH4 (20%), C3H8 (20%), C4H10 (30%), C2H4 (3%), C3H6 (5%), H2S (5%) | – |
| Thermoregulated bath | – | TE2005 | Down to -10°C and up to 80°C. Control accuracy of 0.1°C | Tecnal |
| Mass spectrometer | Gas analysis system | OmniStar | Tungsten (standard) filament. Mass range 1-100 amu. Gas flow rate 1-2 sccm. Qualitative and quantitative gas analysis | Pfeiffer Vacuum |
| Liquid cell | – | – | Optic path length [mm]: 0.3 – 7.5 | LNLS in-house development |
| Potentiostats/Galvanostats | – | N series 273A | – | Autolab EG&G |
| Diffractometer | 4 circle | 424-511.1 | For sample alignment (θ, 2θ, φ, χ) = 0.001° | Huber |
| Electromagnetic coils | Magnetic field | – | Up to 1.5 T | LNLS in-house development |
| Rotary permanent magnet | Magnetic field | – | Up to 0.9 T | Magnetic Solutions |
| Voltage/Current power source | – | BOP-GL 1KW | 4 quadrant bipolar power supply. (0 to ± 50 Vdc) (0 to ± 20 Adc) | Kepco |
| Picoammeter | – | 6485 | – | Keithley |
| Cryostats | – | – | Down to 15 K and up to 420 K | ARS |
| High-pressure cell | High-pressure Diamond anvil cell | Membrane and screw driven | Up to 80 Gpa | LNLS in-house development, Syntek, Princeton |
All beamline controls are done through EPICS (Experimental Physics and Industrial Control System), running on a PXI from National Instruments. The data acquisition is done using a Red Hat workstation with the Py4Syn, developed at LNLS by the SOL group. MEDM (Motif Editor and Display Manager) and Python are used as a graphical interface to display and control the beamline devices.
Users are required to acknowledge the use of LNLS facilities in any paper, conference presentation, thesis and any other published material that uses data obtained in the execution of their proposal.
CEZAR, J. C., SOUZA-NETO, N. M., PIAMONTEZE, C., TAMURA, E., GARCIA, F., CARVALHO, E. J., NEUESCHWANDER, R. T., RAMOS, A. Y., TOLENTINO, H. C. N., CANEIRO, A., MASSA, N. E., MARTINEZ-LOPE, M. J., ALONSO, J. A. & ITIE, J.-P.. Energy-dispersive X-ray absorption spectroscopy at LNLS: investigation on strongly correlated metal oxides. J. Synchrotron Rad. 17, 93-102 (2010). doi:10.1107/S0909049509041119.
An energy-dispersive X-ray absorption spectroscopy beamline mainly dedicated to X-ray magnetic circular dichroism (XMCD) and material science under extreme conditions has been implemented in a bending-magnet port at the Brazilian Synchrotron Light Laboratory. Here the beamline technical characteristics are described, including the most important aspects of the mechanics, optical elements and detection set-up. The beamline performance is then illustrated through two case studies on strongly correlated transition metal oxides: an XMCD insight into the modifications of the magnetic properties of Cr-doped manganites and the structural deformation in nickel perovskites under high applied pressure.
Scientific publications produced with data obtained at the facilities of this beamline, and published in journals indexed by the Web of Science, are listed below.
Galeano-Villar, B. M;Caraballo Vivas, R. J.;Santos, E. C. da S. ;Rabelo Neto, R. C.;Gemini-Piperni, S. ;Finotelli, P. V. ;Checca, N. R. ;Dias, C. S. B.;Garcia, F.. Core-shell Fe@FexOy nanoring system: A versatile platform for biomedical applications, Materials & Design, v.213, p.110303, 2022. DOI:10.1016/j.matdes.2021.110303
Matte, L. P.;Thill, A. S.;Lobato, F. O.;Novôa, M. T. ;Muniz, A. R. ;Poletto, F. S.;Bernardi, F.. Reduction-Driven 3D to 2D Transformation of Cu Nanoparticles, Small, v.18, n.7, p.2106583, 2022. DOI:10.1002/smll.202106583
Anchieta, C. G. ;Assaf, E. M.;Assaf, J. M.. Syngas production by methane tri-reforming: Effect of Ni/CeO2 synthesis method on oxygen vacancies and coke formation, Journal of CO2 Utilization, v.56, p.101853, 2022. DOI:10.1016/j.jcou.2021.101853
Cavichini, A. S.;Orlando, M. T. D.;Fantini, M. C. de A.;Tartaglia, R. ;Galdino, C. W. ;Damay, F.;Porcher, F.;Granado, E.. Enhanced magnetism and suppressed magnetoelastic coupling induced by electron doping in Ca1-xYxMnReO6, Journal of Physics-Condensed Matter, v.34, n.4, p.245803, 2022. DOI:10.1088/1361-648X/ac61b5
Asencios, Y. J. O.;Rodella, C. B.;Assaf, E. M.. Biomethane reforming over Ni catalysts supported on PrO2-ZrO2 solid-solutions, Journal of CO2 Utilization, v.61, p.102018, 2022. DOI:10.1016/j.jcou.2022.102018
Fonseca, R. O. de ;Ponseggi, A. R. ;Rabelo Neto, R. C.;Simões, R. de C. C. ;Mattos, L. V.;Noronha, F. B.. Controlling carbon formation over Ni/CeO2 catalyst for dry reforming of CH4 by tuning Ni crystallite size and oxygen vacancies of the support, Journal of CO2 Utilization, v.57, p.101880, 2022. DOI:10.1016/j.jcou.2021.101880
Aragão, I. B.;Estrada, F. R.;Barrett, D. H.;Rodella, C. B.. Dispersed single-atom Co and Pd nanoparticles forming a PdCo bimetallic catalyst for CO oxidation, Molecular Catalysis, v.526, p.112377, 2022. DOI:10.1016/j.mcat.2022.112377

Português:
Visão Geral da Linha DXAS.
English:
DXAS beamline overview.

Português:
English: