The control processes shown here open new paths to pattern transport from exclusion to preconcentration of charged molecules by selecting the appropriate polymerization strategy and polymerization parameters.
Polymer-mesoporous hybrid materials are an exciting class of materials for a wide range of applications, from controlled release to heterogeneous catalysis or solar cells. The charge and isoelectric point of a membrane play a key role in its mesopore accessibility, and through the control of these features, mesoporous materials are able to regulate transport of charged species. Generating hybrid mesoporous materials by surface-initiated polymerization, thus designing specifically transporting membranes, is a fascinating path toward mimicking controlled transport in nature.
A versatile route toward hybrid membranes is the use of hybrid inorganic mesoporous-polymeric films. Switching of molecular transport through this type of materials can be achieved by inclusion of organic functions, inclusion of charged polymers by adsorption, or grafting.
They offer a tunable, stable, and rigid structure with pores adjustable in the range of several nanometers to hundreds of nanometers, as well as a tailorable surface chemistry that can be adjusted by designing the framework material. Furthermore, they can be deposited on a wide range of substrates at low temperatures and are optically transparent in the visible range, allowing their integration in microelectronic or optoelectronic devices. Polymers, on the other hand, offer a great diversity of function and structure, providing the possibility to multiply functionality by controlling the degree of polymerization. For example, stimuli-responsive mesoporous systems can be designed by grafting species sensitive to physical or chemical solicitations by thoroughly controlling the mesoporous space.
In the particular case of mesoporous thin films, grafting of zwitterionic polyelectrolytes leads to pH-sensitive on−off switchable hybrid membranes; when phosphate-containing polymers are used as a soft building block, films can be designed to be responsive to pH or to the presence of calcium ion. Light-responsive perm-selective membranes have been produced by including polymers containing photolabile groups within mesoporous silica thin films. In addition to on−off switching of transport, a gradual manipulation of transport properties would open a variety of new options from selective molecule sieving to controlled release applications. Performing controlled polymerization in nanoconfined environments permits, in principle, a precise adjustment of charge and isoelectric point, influencing transport characteristics. This could be done through the control of charge density inside nanoconfined pores. The influence of polymerization parameters such as monomer concentration and polymerization time is a central issue that needs to be understood. In recent work, a gradual filling of pores with polymer has been reported but no systematic way to fine-tune these features and to understand their effect on ionic permselectivity has been reported so far.
Recently, Annette Andrieu-Brunsen et al., have described how to control the variation of polyelectrolyte amount in mesoporous aminosilica thin films:
They have explored both temperature- and light-induced polymerization methods. An appropriate choice of the polymerization method and time, allowed controlling the volume fraction of the polymer within the pore. These strategies enabled the gradual control of transport from exclusion to preconcentration. The experimental observations were complemented with predictions from a molecular theory that explicitly considers the polyelectrolyte chains conformational degrees of freedom, the ions and the redox probes, the acid−base chemical equilibria, and the inter- and intramolecular interactions.
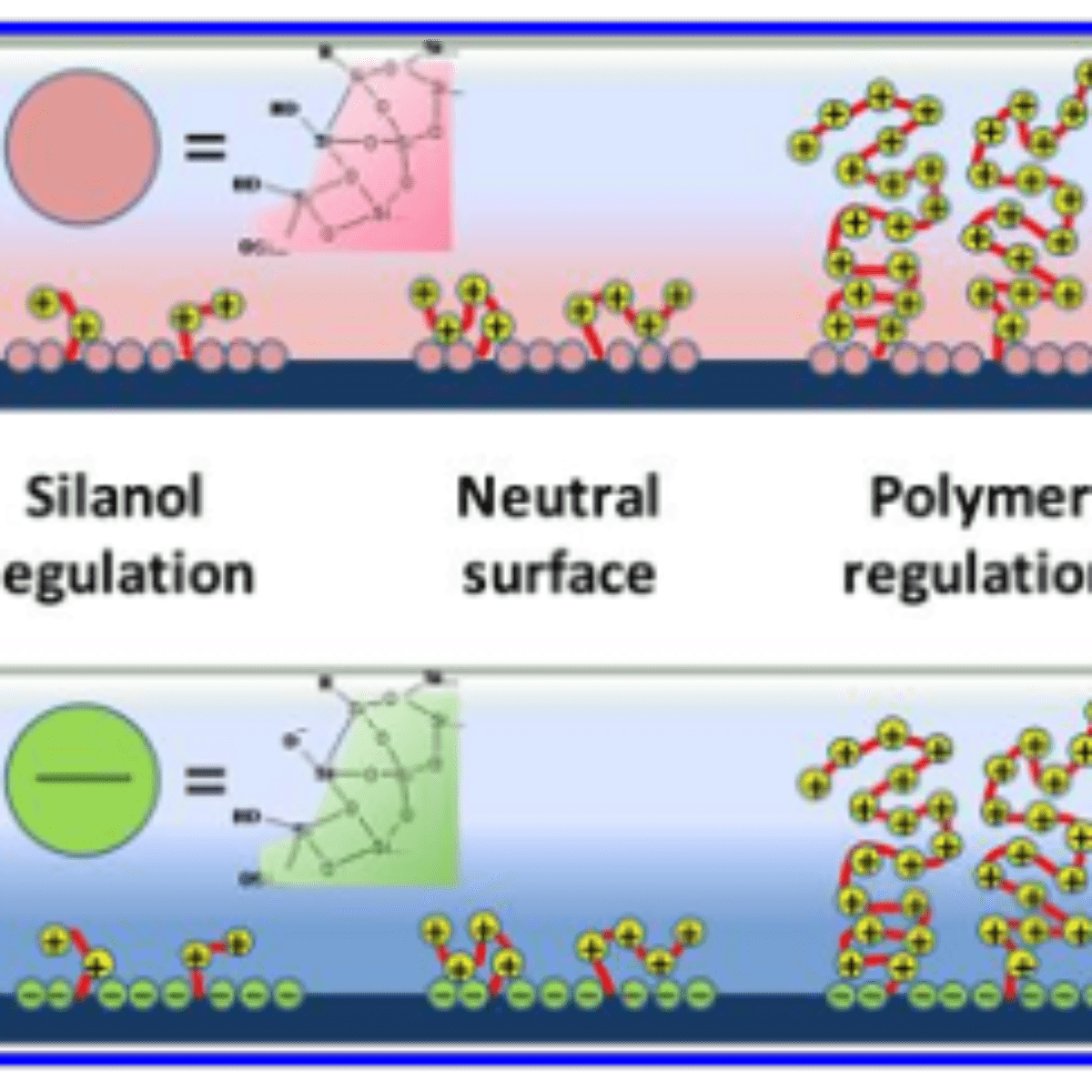
Mesoporous aminosilica films, prepared by co-condensation, show a switch from slightly positively to negatively charged membranes around pH 3−4. The resulting charge distribution within the pores in combination with the small pore diameter results in an exclusion of negatively charged molecules and a preconcentration of positively charged molecules at pH > 4. It has been reported that inclusion of PMETAC brushes with a polymerization degree N ∼ 30 inside the mesoporous amino- silica membrane leads to a completely different behavior (i.e., preconcentration of negative precursors) due to the permanent positive charges of the quaternary amino-function of PMETAC. The authors have achieved a gradual tuning of the transport properties of hybrid mesoporous aminosilica-PMETAC membranes through the precise adjustment of PMETAC density that leads to a gradual adjustment of the positive charge density inside the nanoconfined pore environment, as schematized in Fig. 1. Amino-silica mesoporous film matrices were produced, and an initiator for radical polymerization was grafted to the amino function. The polymerization involved temperature- or light-induced polymerization of METAC+ monomers.
They used mesoporous amino- silica films produced by co-condensation as a platform to build up the hybrid polymeric-inorganic membranes. Films were produced by dip-coating on silicon, glass, or indium tin oxide substrates using Pluronics F127 block copolymer as the structure-directing agent. Films exposed to successive consolidation and extraction.
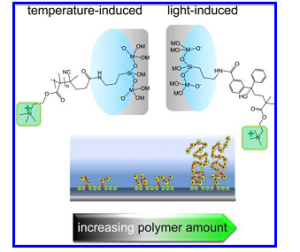
Figure 1. Schematic depiction of the two routes (thermal- and light- induced) with different initiators explored for reaction-time dependent adjustment of PMETAC function density in mesoporous aminosilica films. The transport regulating variables are linked with the ability of adjusting polymerization time and type of polymerization on the amount of permanently positively charged PMETAC brushes, and tuning the polymer interaction with the silanol surface groups that define the pore charge.
In order to manipulate the polymer content and thus the charge density, polymerization time was varied between minutes up to several hours. The presence of processes display organized pore arrays with pores having an ellipsoidal shape due to uniaxial contraction. These functional groups were used as grafting sites for the surface-initiated free radical polymerization of poly 2-(methacryloyloxy)ethyltrimethylammonium chloride) (PMETAC) brushes.
The films were characterized by various techniques including transmission electron microscopy (TEM), cyclic voltammetry, diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS)and small-angle X-Ray scattering (performed at the D10A-XRD2 beamline of LNLS). In order to manipulate the polymer content and thus the charge density, polymerization time was varied between minutes up to several hours. Diffuse Inf DRIFTS measurements confirmed the presence of PMETAC. Light-induced polymerization, with benzophenone as a type II photoinitiator, leads to much higher overall polymer content, as well as a faster polymerization with respect to overall reaction time and achieved polymer amount under the applied experimental conditions.
The combination of experiments and theory showed that ionic perm-selectivity in mesoporous hybrid thin films can be adjusted gradually by adjusting polyelectrolyte content, either by changing the polymerization method or by varying the polymerization time. As sketched in Figure 2, three regions can be clearly determined for both acidic and alkaline media:
(a) $ n_{METAC} / n_{NH_2} < 2 $ (i.e., an occupied pore volume of F% < 25%), in which a combination of the surface charge modulated by the silanols, residuals of amino/ammonium functions, and some METAC groups are responsible for the selective transport of ions, depending on pH (i.e., the system is selective to cations at high pH and to anions at low pH),
(b) $ n_{METAC}/ n_{NH_2} \approx 2−5 $, with neutralized pores through which transport of cations or anions can take place, and no exclusion is observed,
(c) $ n_{>METAC} / n_{NH_2} > 5 $, in which the transport of cations dramatically decreases, and anion transport or preconcentration can be controlled. However, the observed behavior is more complex and smaller residual non perm-selective currents can be due to interfacial effects such as adsorption.
The control processes shown here open new paths to pattern transport from exclusion to preconcentration of charged molecules by selecting the appropriate polymerization strategy and polymerization parameters. Interestingly, pore volume effects do not seem to play a central role; this may be due to a compensation between preconcentration and volume reduction effects.
In addition, the access of anions into a pore that is completely filled by polymer suggests that the polymer is permeable to the ions per se and the ion transport is mostly.
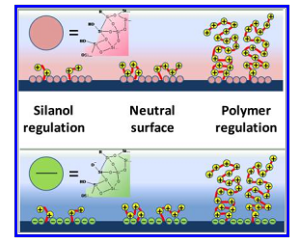
Figure 2. Schematic summary of the behavior of the polymer-modified silica surface. The upper scheme represents acidic conditions (pH < 3) in which the surface is covered with silanol or protonated silanol groups. The lower figure represents alkaline media (pH > 7), presenting silanolate surface groups. The polymerization degree, N, increases from left to right; regions of different charge control regime are indicated.
The possibility of using different polymerization strategies to control polymer content or macroscopic positioning opens a variety of new paths for transport tuning, and should be applicable to a wide variety of hybrid membrane systems integrated in lab-on-chip or electronic devices. In addition, the possibilities of controlling the polymer loading within a mesopore by irradiation opens interesting avenues in the lithographic patterning of mesoporous materials for micro- fluidics.
Source: Annette Andrieu-Brunsen, Sebastien Micoureau, MarioTagliazucchi, Igal Szleifer, OmarAzzaroni, and Galo J. A. A. Soler-Illia, Mesoporous Hybrid Thin Film Membranes with PMETAC@Silica Architectures: Controlling Ionic Gating through the Tuning of Polyelectrolyte Density. Chem. Mater., 2015, 27 (3), pp 808–821. DOI: 10.1021/cm5037953
This immediately suggests the hypothesis that, since very few T4SS have been characterized to date, T4SS-mediated bacterial killing may not be restricted to the Xanthomonadaceae family, and may in fact be a more widespread phenomenon.
These observations open up possibilities for the study of protein folding and provide a new interpretation to explain the nature of the cooperative behavior of proteins during folding reactions.