Research proposes new mechanism for Suzuki-type C-C homocoupling reaction catalyzed by palladium nanocubes
The production of chemical compounds from simpler organic molecules is of great importance for various industrial processes. It is based on the bonding between carbons of the precursor organic compounds, aided by catalysts (typically transition metals). These reactions make it possible to obtain natural and synthetic substances for the development of new materials, such as polymers and pharmaceuticals.
In particular, the so-called carbon-carbon (CC) cross-coupling reactions, in which two different precursor molecules are bound to form the final chemical compound, are of such importance that their development granted the 2010 Nobel Prize in chemistry to researchers Richard F. Heck, Ei-ichi Negishi and Akira Suzuki.
Another type of coupling reaction is the C-C homocoupling reaction, in which two similar precursor molecules bond forming a symmetrical final compound. These reactions began to gain prominence due to their similarities to cross-coupling reactions, which allowed the optimization and development of new catalysts for both mechanisms.
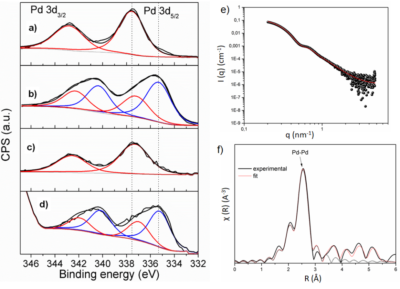
Figure 1. XPS spectra of the Pd-NCs catalyst under different reaction conditions (a-d), SAXS (e) and XAS (f) analyses.
Most of the studies on the homocoupling reactions of boronic acids (or Suzuki-type) were carried out with the use of palladium (Pd) organometallic catalysts and very little is known about the use of Pd nanoparticles as catalysts for these reactions.
The development of Pd nanoparticles can provide similar, or even higher, selectivity and yields, and allow for more efficient industrial processes. For example, nanoparticles have a high surface area by volume, substantially reducing the amount of catalyst used in the reactions. This is especially important in the case of Palladium, a rare and expensive metal.
Thus, Welman C. Elias et al. [1] used the facilities of the Brazilian Synchrotron Light Laboratory (LNLS) to study the mechanism of the homocoupling reaction of trans-2-phenylvinylboronic acid catalyzed by palladium nanoparticles (Pd-NCs) in the presence and absence of base.
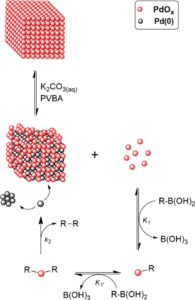
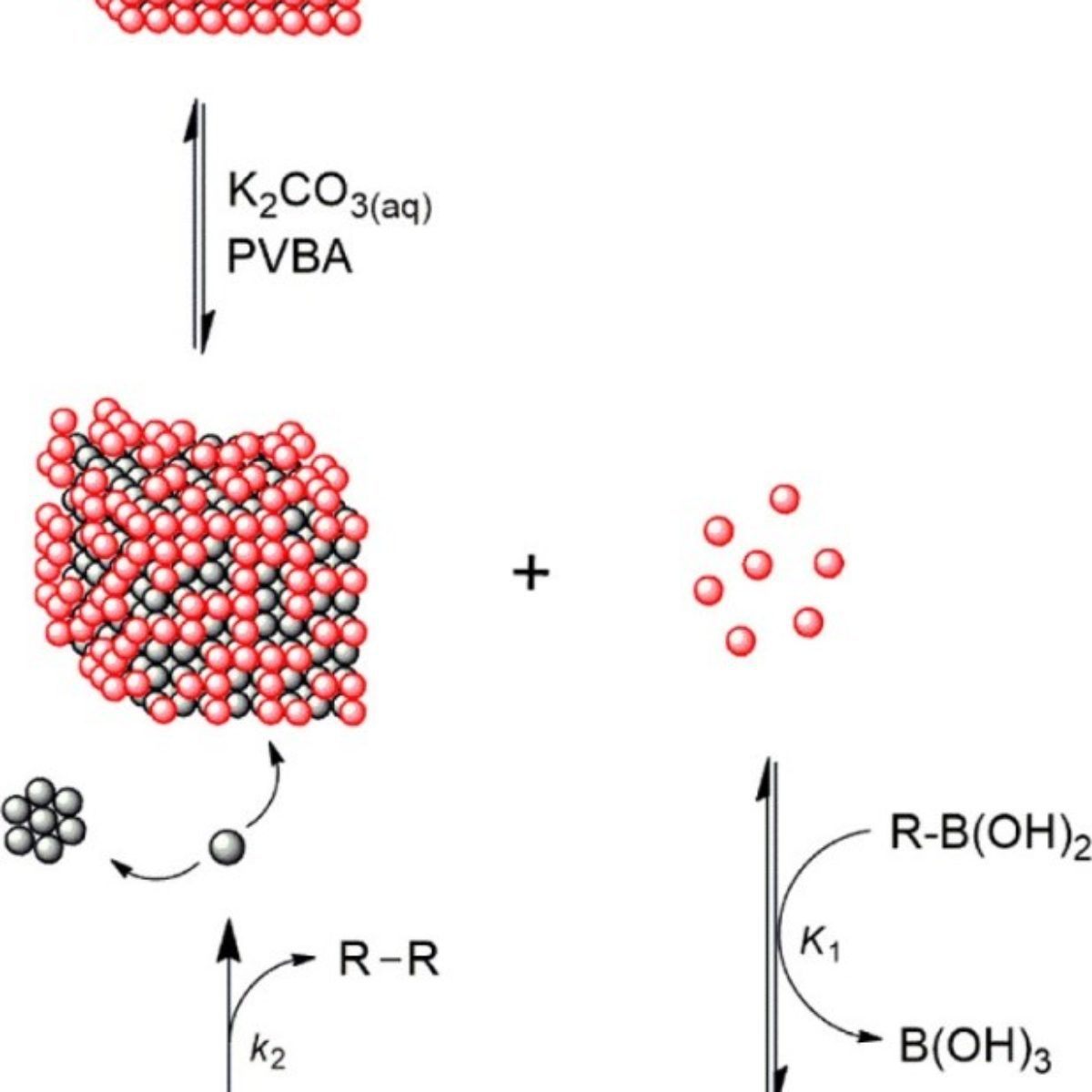
Figure 2. Homocoupling reaction of trans-2-phenylvinylboronic acid (PVBA) catalyzed by Pd-NCs.
The nanoparticles were characterized by X-Ray Photoelectron Spectroscopy (XPS), Small Angle X-ray Scattering and X-ray Absorption Spectroscopy at SAXS1 and XDS beamlines (Figure 1).
Associated with kinetic studies and catalyst poisoning, the data obtained in the LNLS have shown that palladium oxide ($\rm PdO_X$) is the active species of the trans-2-phenylvinylboronic acid homocoupling reaction, and originates from leaching of the atoms that make up the surface of the Pd nanoparticle. The leaching of these oxides of palladium is favored by the joint effect of the base and the boronic acid, with the Pd nanoparticle acting as a reservoir of the active species, as shown in Figure 2.
Based on these data, the authors propose for the first time in the literature a mechanism in which the homocoupling reaction of a boronic acid is catalyzed by species of $\rm PdO_X$ via an oxo-palladium-type transmetalation pathway. Such a contribution allows new catalysts to be rationally designed to improve the efficiency for both C-C homocoupling and cross-coupling reactions.
Source: [1] Elias, W.; Signori, A.; Zaramello, L.; Albuquerque, B.; Oliveira, D. C.; Domingos, J. B. Mechanism of a Suzuki-Type Homocoupling Reaction Catalyzed by Palladium Nanocubes. ACS Catalysis, v. 7, p. 1462-1469, 2017. DOI: 10.1021/acscatal.6b03490
Research investigates the impact of oxidation on electronic and vibrational properties
Research analyzes molybdenum catalysts doped with transition metals