New catalyst shows a potential for industrial applications requiring durability and high thermal stability.
If Au nanoparticles can be stabilized a number of potential applications become viable. For example, most pollution from automobiles is emitted in the first 5 minutes after start-up, while the engine is still being warmed. This is because platinum group metals that constitute the active phase of current automotive catalysts are poisoned by CO at lower temperatures. For this reason, light-off, the temperature at which the catalytic reaction is initiated, in current catalytic converters occurs between 200 and 300°C resulting in light-off pollution. Nano-Au catalysts, however, light-off well below these temperatures but deactivate rapidly due to the poor thermal stability of nano-Au as the catalytic activity is strongly dependent on Au particle size for many reactions.
In spite of many attempts in the last 20 years, a sinter resistant , nano-Au catalyst with stable Au nanoparticles, has not been developed.
Recently, a group of scientists from Brazil and South Africa has reported a new way to produce nano-Au stability in a definitive , reproducible and generally applicable way (3). Phase pure titanium dioxide rutile nanorods with a specific morphology (radially aligned nano rutile) were prepared using a mild hydrothermal synthesis at ambient pressure. Alignment of the rutile nanorods was verified by electron diffraction, transmission electron microscopy and in situ X-Ray powder diffraction with each nanorod having the same crystallographic orientation. Then nanoparticulate Au was deposited onto the rutile nanorods with theoretical loadings of 1.2%, 5% and 6% Au by mass using deposition–precipitation with urea. Rietveld refinement and pair distribution function analysis confirmed Au loadings of 1%, 3.7%, and 5.4%, respectively.
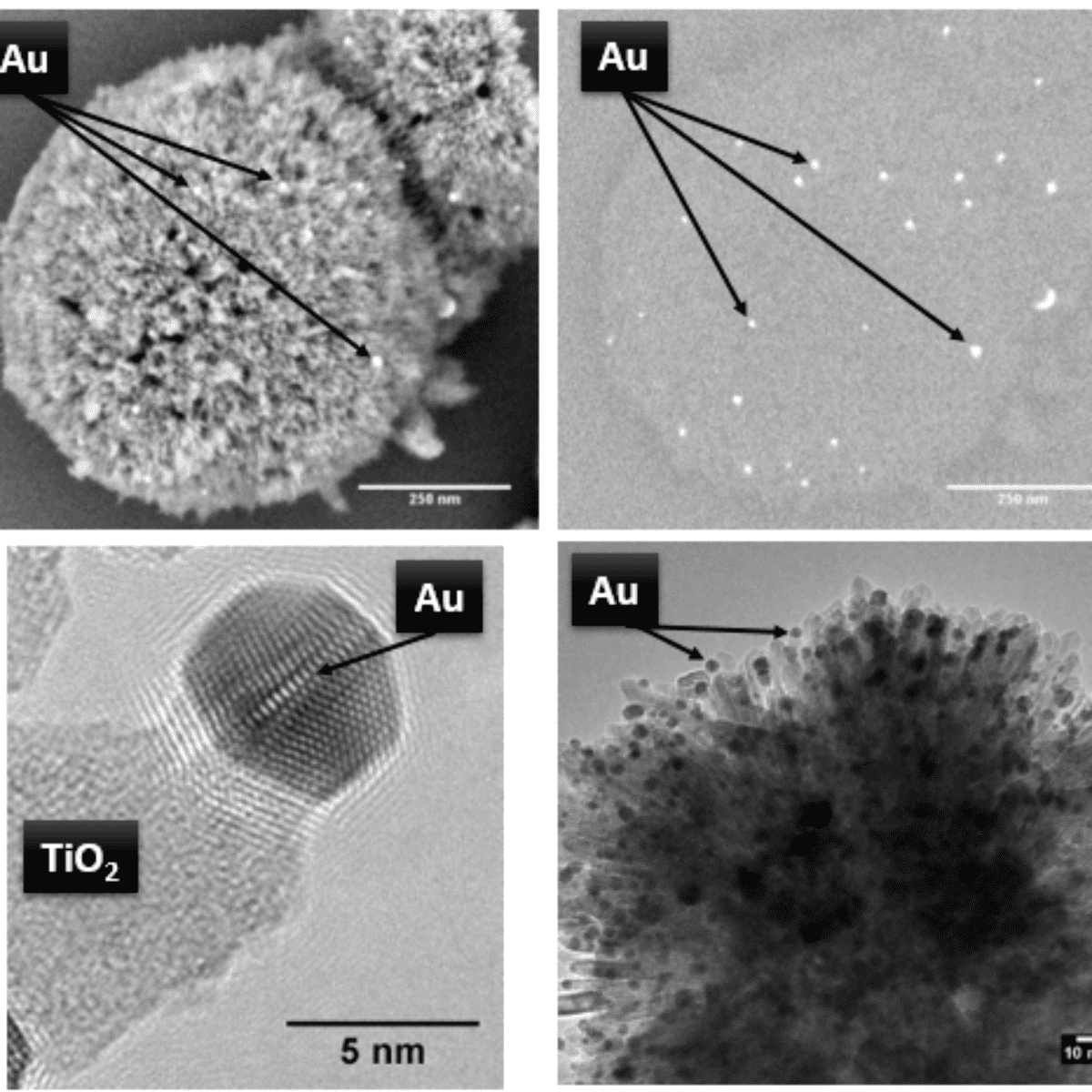
As seen in Fig. 1, various electron microscopy and X-Ray techniques confirmed the presence of the Au nanoparticles isolated on the nanorods both before and after thermal treatments, under synthetic air to 810°C. Prior the treatment, the catalysts were activated under hydrogen flow at 300°C for 1 hour. The results are clearly observable on Figure1. Thus, the assembly of the nanorods in an anisotropic fashion provide enhanced defect sites, which aid Au stability. The presence of Au nanoparticles at the tips of the nanorods confirms this finding. Undercoordinated defect sites located on the tips of the nanorods act as binding sites for the Au nanoparticles, effectively isolating them at the tips of the structure even after thermal treatment to 810°C.

Fig. 1: Scanning electron microscopy (top left) of the catalyst after activation with the corresponding back scattered image (top right) highlighting the Au nanoparticles as bright white flashes. The images show the dispersion of Au nanoparticles which are isolated on the periphery of the structure after activation of the catalyst under hydrogen. High-resolution transmission electron microscopy (bottom left) after activation of the catalyst : the image shows the well-defined structure and distinct surface geometry of the Au nanoparticles at the tips of the nanorods as well as showing evidence of strong binding between the titania and the Au nanoparticles. Transmission electron microscopy image (bottom right) of the catalyst after in situ X-ray powder diffraction measurements for over 200 hours and holding at 800°C for 5 hours confirmed the minimal growth of the nano-Au.
In-situ X-Ray powder diffraction provided quantitative information about the catalyst structures when the temperature was increased : this is illustrated in Fig.2 which shows the very small increase of Au-nanorods catalysts compared to the commercial ones. One should mention that catalytic testing was undertaken with the use of the CO oxidation reaction. The reaction served two purposes. Firstly, CO oxidation is an industrially important reaction in various applications and secondly, CO oxidation is highly structure sensitive with respect to the Au nanoparticle size. Thus, the reaction acts as an ideal probe to aid in characterizing the catalyst via catalytic data. Thermal cycling under harsh conditions, long duration, high temperature, an oxidizing atmosphere, and multiple cycles is important to determine a catalysts durability.

Fig. 2: Au crystallite sizes of the commercial $ \rm Au-TiO_{2}$ and Au-rutile nanorods catalysts determined from Rietveld refinement from in situ X-Ray powder diffraction.
Catalysts were therefore thermally cycled to 700°C. Both Au-nanorods and commercial Au–TiO2initially show high activity with light-off temperatures from 20°C, as they contain well dispersed, small Au-nanoparticles able to readily catalyse the reaction. As temperatures increased the well-documented effect of substantial Au sintering takes place for the commercial $ \rm Au-TiO_{2}$ catalysts resulting in a complete deactivation after a short period of thermal exposure (1 cycle at 700 °C).
In conclusion, the new catalyst shows a potential for industrial applications requiring durability and high thermal stability. In addition, it maintains CO light-off temperature well below that of any current platinum group metal based catalyst even after rigorous thermal cycling at 800 °C.
The X-Ray experiments were performed with a Cu source or with synchrotron radiation at the XPD beamline in LNLS (8 KeV) or at the Advanced Photon Source (58 KeV). The electron microscopy data were collected at the University of the Witwatersrand, South Africa and at the LNNano Laboratory in Campinas (thanks to Erico Teixeira Neto from LNNano).
[1] M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, Au catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide, J. Catal.,1989, 115, 301.
[2] A. Cho, Connecting the dots to custom catalysts, Science, 2003, 299, 1684.
[3] Source: Dean H. Barrett, Michael S. Scurrell, Cristiane B. Rodella, Beatriz Diaz, David G. Billing and Paul J. Franklyn. . Achieving nano-gold stability through rational design, Chem. Sci., 2016,7, 6815-6823. DOI: 10.1039/C6SC01597B. See also D. H Barrett and P. J. Franklyn, US 9,352,300 B2
Hexagonal-shaped Cu2Te nanodisks self-organize into a network of ribbons fully embedded in rr-P3HT thin films.
The first real-time in situ XANES/EXAFS study of the formation kinetics of Mo-doped and undoped fe oxide NPs.