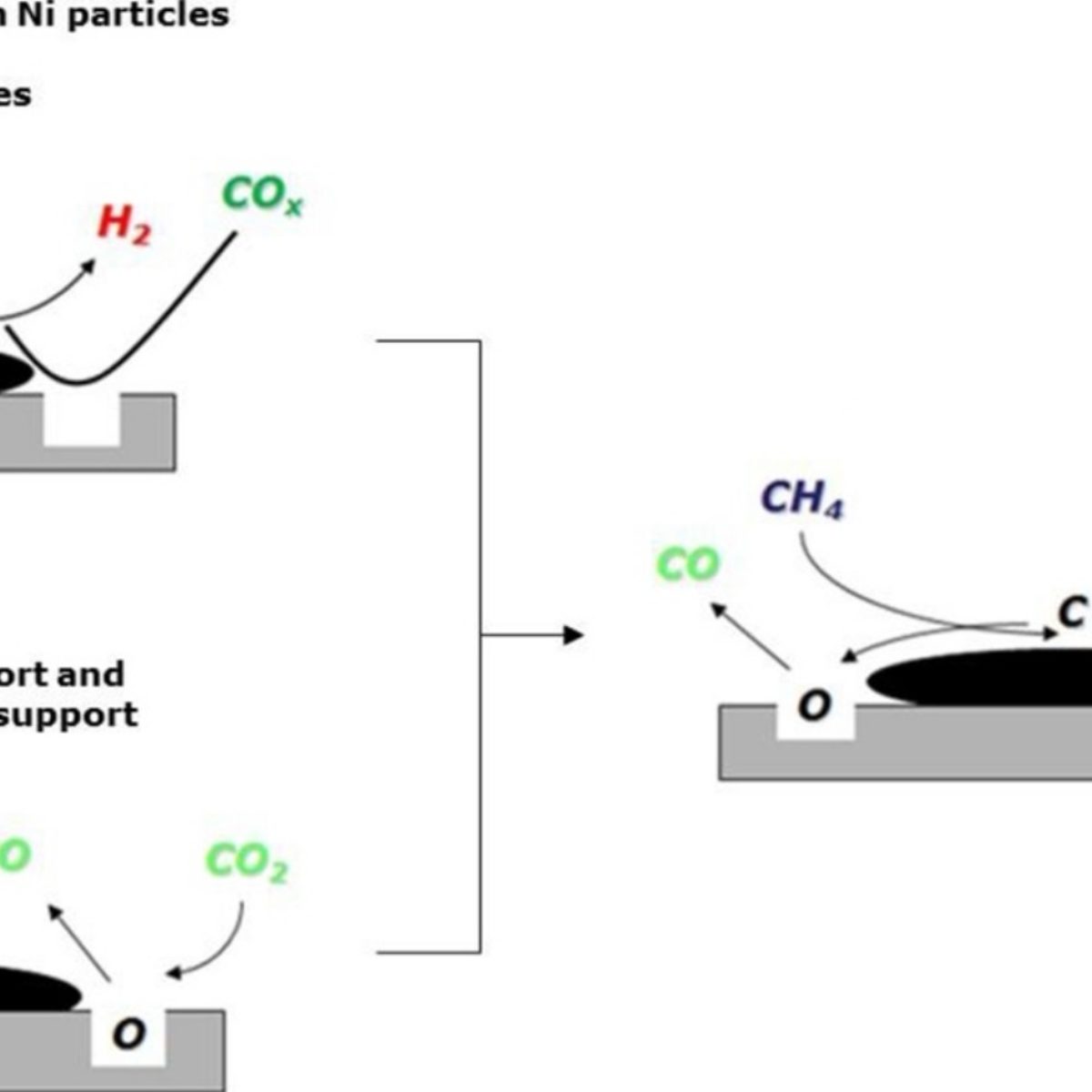
Research analyzes the deactivation of nickel catalysts in dry reforming of methane
Hydrogen gas ($\rm H_2 $) is one of the alternatives to fossil fuels since its combustion has as final product only water vapor. A promising way to produce hydrogen is from the so-called biogas: methane ($\rm CH_4 $) and carbon dioxide ($ \rm CO_2 $) originated from the fermentation of organic matter in anaerobic environments, such as landfills.
One possible process for this transformation is the dry reforming of methane (DRM), where $\rm CO_2 $ and $\rm CH_4$ in the biogas react (in the presence of a catalyst) yielding a mixture of $\rm H_2$ and carbon monoxide ($\rm CO$) known as the synthesis gas.
A small fraction of the biogas is already used for the production of electricity through its combustion, but most of it is released into the atmosphere where it contributes significantly to global warming. Thus, through the DRM process, it is possible to obtain, from two greenhouse gases, both an alternative energy source in the form of hydrogen and important precursors for the production of more complex substances in the chemical industry.
However, for this technology to be economically feasible, it is still necessary to develop an active catalyst which is resistant to the deposition of carbon under the operating conditions used in biogas reform. For this reason, Raimundo C. Rabelo-Neto et al. used the facilities of the Brazilian Synchrotron Light Laboratory (LNLS) to study the performance of nickel catalysts supported on different oxides during the dry reforming of methane.
The catalysts were produced from a perovskite-type oxide precursor $\rm LaNiO_3$, both pure and supported on alumina ($\rm Al_2 O_3$) and on an oxide based on cerium and silica ($\rm CeO_2-SiO_2$). Through analyses that included in situ X-ray diffraction performed on the XPD beamline, the researchers monitored the structural and surface changes of the catalysts during the reactions.
The group observed that the largest amount of carbon formed in the unsupported $\rm LaNiO_3$ catalyst, while only one third of that amount was deposited on the catalyst supported in $\rm Al_2 O_3$. Finally, there was virtually no carbon formation on the catalyst supported in $\rm CeO_2-SiO_2$. The researchers attributed this effect to the higher capacity of ceria-based support in storing and releasing oxygen. The increased oxygen mobility in this compound promotes the removal of carbon, increasing the stability of the catalyst.
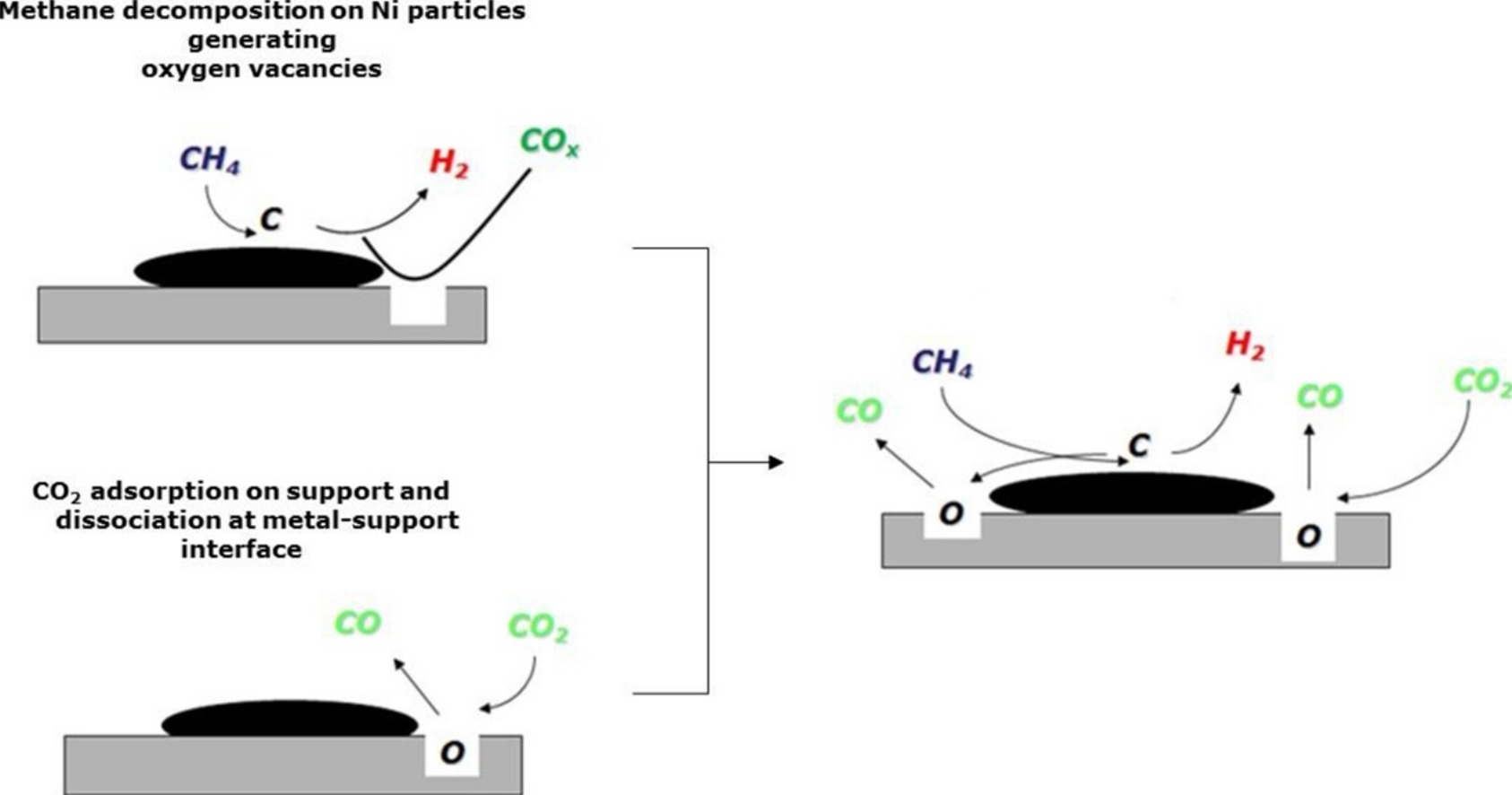
Figure 1: Scheme for DRM with the nickel catalyst supported in $\rm CeO_2-SiO_2$
Source: R. C. Rabelo-Neto, H. B. E. Sales, C. V. M. Inocêncio, E. Varga, A. Oszkoc, A. Erdohelyi, F. B. Noronha, L. V. Mattos. CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Applied Catalysis B: Environmental 221 (2018), 349-361. DOI: 10.1016/j.apcatb.2017.09.022
Research investigates the addition of ceria on the activity of catalysts for the water-gas shift reaction
A new research demonstrates a direct and selective way to investigate 5f electrons in actinide compounds as well as their interaction with other valence electrons