Research investigates the addition of ceria on the activity of catalysts for the water-gas shift reaction
Catalysts are substances that promote and accelerate chemical reactions without being consumed during the process and are widely used in industrial processes to produce various chemicals.
Catalysts based on copper nanoparticles dispersed in an oxide support benefit various reactions, such as the synthesis of methanol, the alcohol dehydrogenation, or the water gas shift (WGS) reaction which is one of the main processes for hydrogen production on an industrial scale. In this reaction, carbon monoxide reacts with water to produce carbon dioxide $ \rm CO_2 $ and hydrogen gas $\rm H_2$.
The activity of catalysts in these reactions is sensitive both to the morphology of the metal nanoparticles and to their interactions with the support. Therefore, Paula C. P. Caldas et al. [1] used the facilities of the Brazilian Synchrotron Light Laboratory (LNLS) to investigate how the catalytic activity and the properties of the active sites on the surface of copper nanoparticles can be modified by its size and its interaction with ceria ($ \rm CeO_2 $).
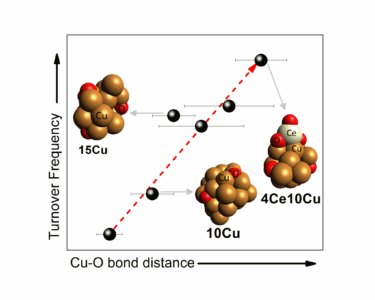
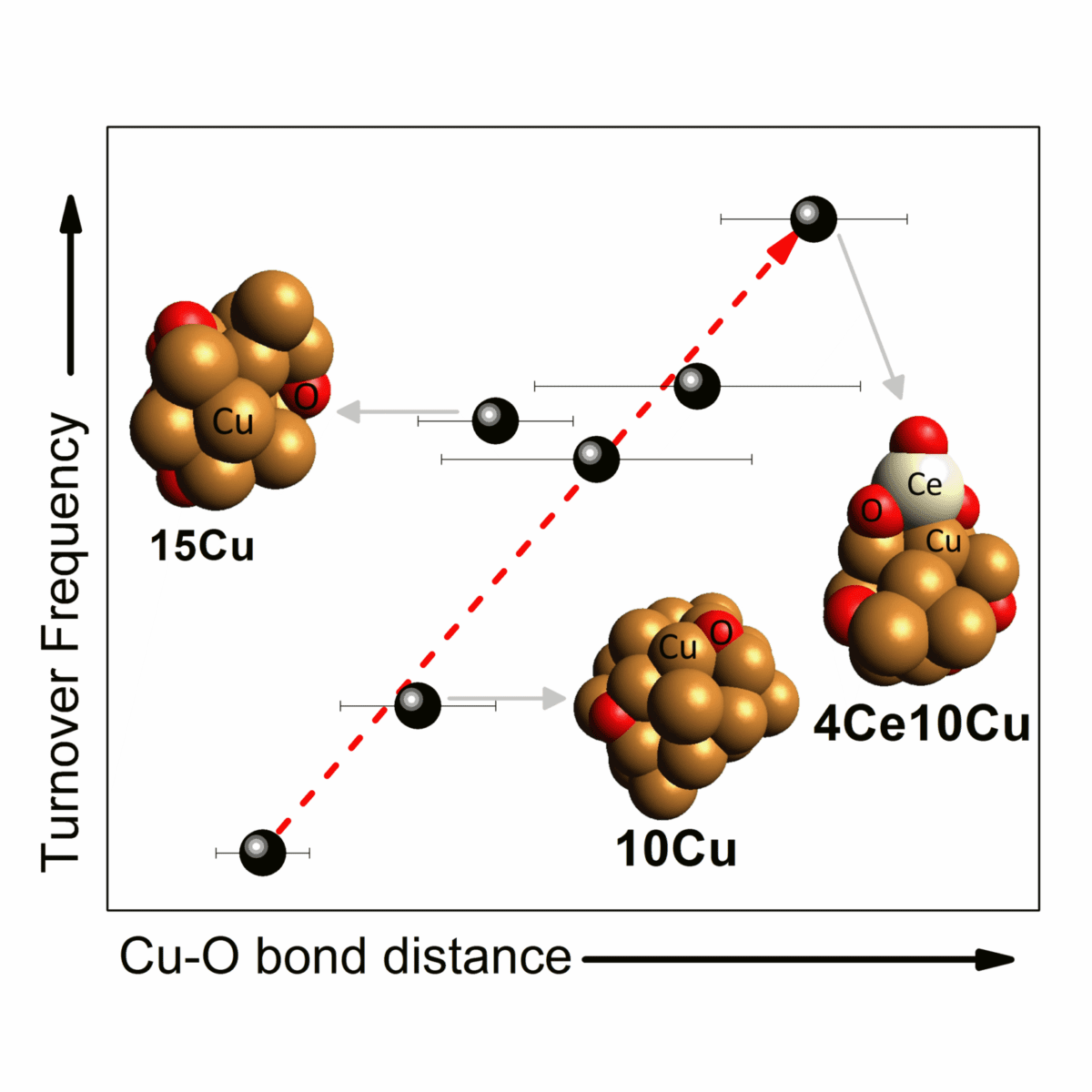
Figure 1: Correlation between the bond length of $\rm Cu\, — O$ and the catalyst turnover frequency (TOF) for the catalysts analyzed under WGS conditions with different proportions of copper and ceria.
In this study, the researchers used the WGS reaction as a model for the analysis of the activity of the copper catalysts, supported in $\rm \gamma-Al_2O_3$, with different proportions of the metal. The experiments included X-ray absorption analyses (XANES and EXAFS) performed on LNLS’ XAFS1 beamline.
The group observed that the larger the size of the copper nanoparticles, the longer the bond length between copper and oxygen atoms $\rm Cu\, — O$ in the catalyst, and consequently the greater its catalytic activity. This happens because a longer bond length corresponds to a lower bonding energy between the atoms, which leads to a higher electron density for the copper on the surface of the catalyst and a higher reactivity for the oxygen. Thus, there is an increase in the rate of intermediate reactions of the WGS process.
Furthermore, ceria was added in different proportions to the copper catalyst, which also provided an increase in the $\rm Cu\, — O$ bond length without increasing the size of the nanoparticles. For example, for the catalyst containing $10\, wt\, \%$ copper ($\rm 10Cu$), the bond length increased from $ 1.890 ± 0.02$ Å to $ 1.941 ± 0.012$ Å with the addition of $4\, wt\, \%$ ceria ($ \rm 4Ce 10Cu $). The correlation between the bond length $\rm Cu\, — O$ and the turnover frequency (TOF) for the catalysts analyzed under WGS conditions with different copper and ceria proportions is shown in Figure 1.
The experimental results presented by the researchers indicate that the addition of ceria adjusts the $\rm Cu\, — O$ bond length, which determines the reaction rate of the WGS process and demonstrates that the fine structure of the active sites at the interface of the nanoparticles must be determined for the understanding of the effects of nanoparticle size and support on the activity of the catalysts used in this type of reaction.
Source: [1] Paula C. P. Caldas, Jean Marcel R. Gallo, Alejandro Lopez-Castillo, Daniela Zanchet, and José Maria C. Bueno. The Structure of the Cu–CuO Sites Determines the Catalytic Activity of Cu Nanoparticles. ACS Catal., 2017, 7 (4), pp 2419–2424. DOI: 10.1021/acscatal.6b03642
A new research demonstrates a direct and selective way to investigate 5f electrons in actinide compounds as well as their interaction with other valence electrons
Research proposes new mechanism for Suzuki-type C-C homocoupling reaction catalyzed by palladium nanocubes