The authors have demonstrated the effective biological activity of a noncomplex nanomaterial against susceptible and antibiotic-resistant bacteria without significant cytotoxicity.
Infections and several diseases caused by resistant micro-organisms in which the conventional treatment often fails result in prolonged illness and greater risks of death. The inappropriate and irrational use of antibiotics and antimicrobial drugs can lead to resistant microorganisms and provide them with favorable conditions to emerge, spread, and persist. According to the World Health Organization (WHO), a high percentage of hospital-acquired infections are caused by highly resistant bacteria and 440 000 new cases of multidrug-resistant tuberculosis emerge annually, causing at least 150 000 deaths.
In this context, the emergence of different antibiotic-resistant and multidrug-resistant pathogenic bacteria has boosted the development of new technologies and alternative materials to overcome this public health problem. One of the possible strategies is based on the production of antimicrobial and antiadhesive surfaces.
Mesoporous silica nanoparticles have attracted great attention due to the possibility of shape tuning as well as for the entrapment/encapsulation of antitumor agents, markers, proteins, genes, and DNA where a tailored delivery system can be achieved.
Smart and Efficient Alternative
Recently, L. B. Capeletti et al. (1) demonstrated the encapsulation of tetracycline (TC) antibiotic into silica nanoparticles. Although silica nanoparticles have been successfully developed as a bactericidal and virucidal material, this is the first report of composites, which are efficient against susceptible and even doubly resistant bacteria without significant mammalian cell cytotoxicity. It was found that the composites are quasi-spherical nanoparticles with average size of approximately 60 nm. The average size as well as the size distribution were also investigated through small-angle X-ray scattering (SAXS) in solution at the SAXS1 beamline of the Brazilian Synchrotron Light Laboratory (LNLS) and by transmission electron microscopy (TEM) at LNNano.
Bacterial Properties
The nanoparticles’ bactericidal efficiency was tested against resistant and susceptible E. coli and compared with free TC or a mixture of TC and ampicillin (Amp) antibiotics. The bacteria were incubated with either nanoparticles or free antibiotic during 5 h, and the colony growth was examined by plating incubated mixtures on nutrient agar plates. All incubations were performed using a known mass of antibiotic and/or silica. Figure 1 presents the percentage of surviving susceptible and resistant bacteria in the absence and in the presence of the composites and/or antibiotics.
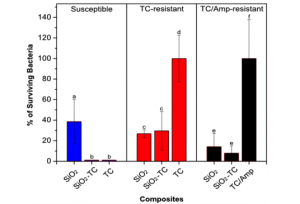
Figure 1. Antimicrobial activity of silica nanoparticles and silica-tetracycline composites against susceptible (blue bars), TC-resistant (red bars), and TC/Amp-resistant (black bars) E. coli. Bar representation indicates the percentage of surviving bacteria colonies for each test.
The percentage of surviving colonies for susceptible E. coli (blue bars) was 38.5 ± 21.4% for $ \rm SiO_2$, and practically no colonies were observed for $ \rm SiO_2-TC$ and free-TC. Comparative antimicrobial activity of $ \rm SiO_2$ and free-TC against susceptible E. coli was confirmed by flow cytometry. In parallel, the percentage of surviving colonies for TC-resistant E. coli (red bars) for $ \rm SiO_2$ and $ \rm SiO_2-TC$ was 32.3 ± 3.6% and 34.7 ± 18.3%, respectively, while no bacteria inhibition was seen for free-TC.
Furthermore, doubly resistant bacteria were tested where TC and Amp have shown no bactericidal effect against these bacteria. In this case, the results are similar to those obtained for TC-resistant bacteria. The percentage of surviving colonies for TC/Amp-resistant E. coli (black bars) for $ \rm SiO_2$ and $ \rm SiO_2-TC$ was 14.2 ± 12.1% and 7.9 ± 6.3%, respectively, while a mixture of TC and Amp was not able to prevent bacteria growth.
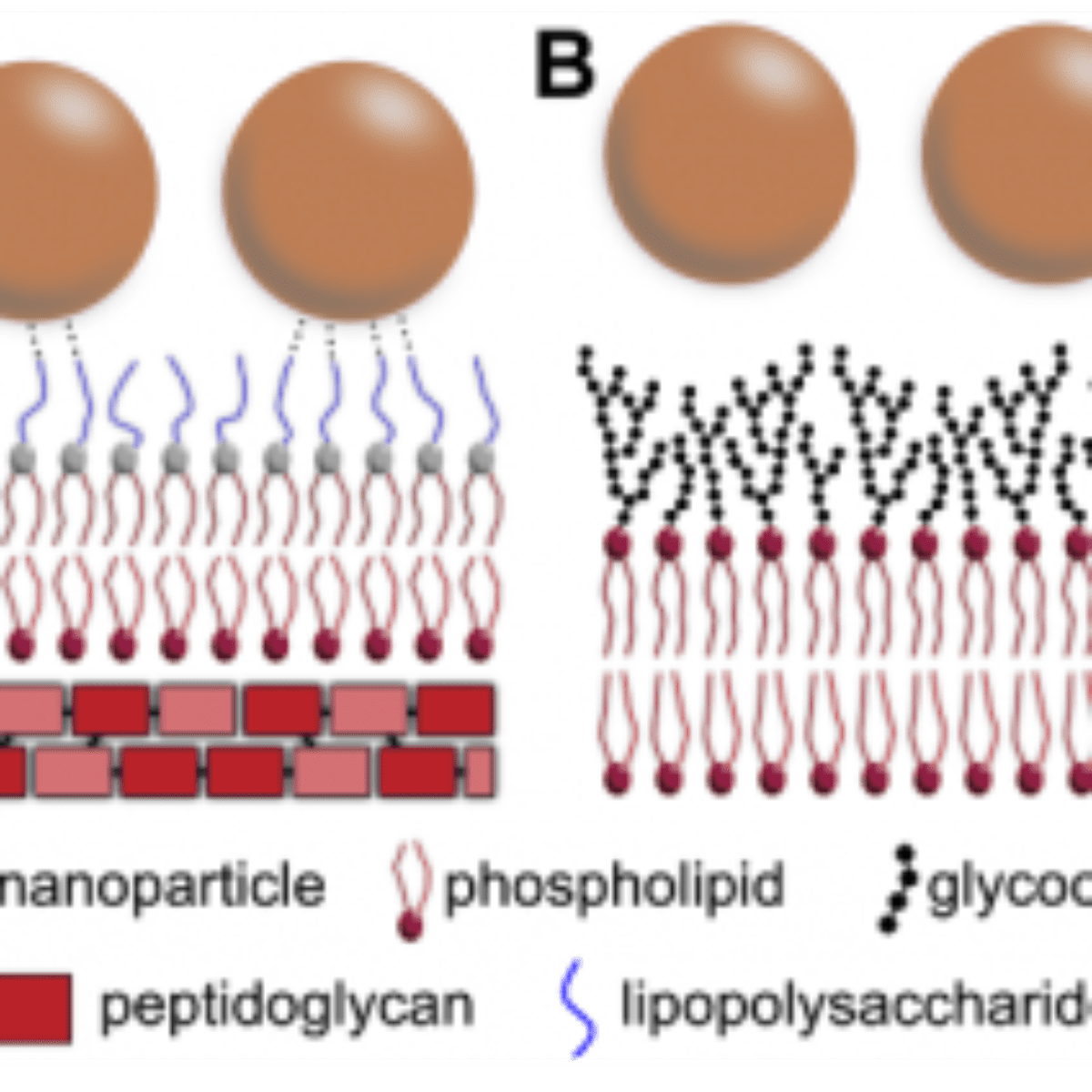
This behavior highlights the bactericidal effect of these nanoparticles to both susceptible and even multiple resistant E. coli, in which the free-antibiotic could not efficiently kill the bacteria. This occurs because the nanoparticle itself can also kill the bacteria. These microorganisms present a cell wall, which consists of an outer membrane composed of lipopolysaccharides, phospholipids, and transmembrane proteins, with an underlying peptidoglycan layer (Figure2).
The outer lipopolysaccharide layer is composed by units of the saccharides abequose, mannose, rhamnose, galactose, and glucose. Based on the results and membrane architecture reported above, the authors propose that nanoparticles can interact with the lipopolysaccharides through hydrogen bond formation between the saccharides and hydroxyl groups present on the silica surface. As the nanoparticles are in contact with the bacteria in solution under shaking, the probability of interactions through functional groups is increased. Also, the constant shaking can help the nanoparticles to reach and destabilize the peptidoglycan layer, since it has different chemical groups, which can interact with the silica surface. Although for distinct systems, several works have also demonstrated this behavior related to hydroxyl groups.
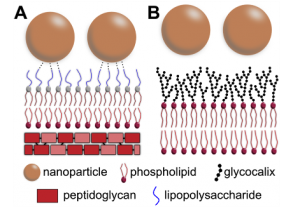
Figure 2. Schematic representation of the interaction between the silica-tetracycline nanoparticles and membranes. E. coli Gram negative bacteria (A) and mammalian cells (B) have different membrane structures that reflect different behavior when they are in contact with nanoparticles. Possible hydrogen bonds, which supposedly increase the nanoparticle-membrane interaction, are schematically represented in panel (A).
Cytotoxicity Tests in Human Cells
As both susceptible and resistant bacteria could be effectively killed by the nanoparticles, cytotoxicity tests were performed to investigate the effect of the nanoparticles in human cells. Figure 3 shows images of HEK 293t cells in the absence (control sample, A-C) and in the presence of the $ \rm SiO_2-TC$ composites (nanoparticles presence, D-F) after 12 h of incubation. The nuclei are shown in panels (A) and (D) for the control and $ \rm SiO_2-TC$ composites, respectively, and reveal similar characteristics since their forms and edges are retained. No morphological damage, blebbing, or ruffling of the nuclear membrane was observed as may occur in case of apoptosis or necrosis. The cytoplasm was represented here by mitochondria labeling, and it was possible to observe the maintenance of mitochondrial characteristics. Mitochondrial responses to toxic impact depend on the type of compound, on its concentration, and on the specific mitochondrial function that is acted. Most mitochondrial dysfunctions are represented by alterations in mitochondrial membrane potential or in reductive activity of enzymatic oxidoreductases.
These results indicate that the cells were not significantly influenced by the presence of nanoparticles within the first 12 h of experiment. It is only after 24 and 48 h, that one observes a reduction in the cell growth rate in the wells containing the nanoparticles, if compared to the control and free-TC wells. These results suggest that, for a short time period, up to 12 h, no toxicity or growth inhibition was observed, and this opens a possible therapeutic time-window especially for external use of the nanoparticles.
The results presented here show nanoparticles with efficient antibacterial properties against susceptible and resistant bacteria with no significant toxicity to mammalian cells. This behavior can be explained through their action mechanism in the bacteria. Frequently, chemical substances, such as antibiotics, can affect the bacteria cell wall while mammalian cells are preserved. The composition of these membranes is different, and the bacteria are normally killed through the hydrolysis of bonds between the saccharides units, which constitute the peptidoglycan skeleton. If the bacteria have their wall components damaged, they lose mechanical resistance and may die from cellular disruption. It is generally promoted by osmotic stress, since the cells’ swelling tendency in the usual hypo-osmotic surrounding is no longer counterbalanced by the wall. Also, on the bacteria, there is an outer membrane of lipopolysaccharides, which can interact with the nanoparticles through hydrogen bonds of silica surface hydroxyl groups as represented in Figure 2A.
As the mammalian cells do not have the same peptidoglycan wall, they may not be killed in the same way as the bacteria. The cells have an external layer named glycocalyx, which confers protection and represents a highly functional selective filter (Figure 2B).
In this study, the authors have demonstrated the effective biological activity of a noncomplex nanomaterial against susceptible and antibiotic-resistant bacteria without significant cytotoxicity. Further, the material developed here is cost-effective and of controllable fabrication, and these findings could have a significant relevance since it is imperative to find alternative strategies to overcome bacterial resistance problems.
Source:
(1) Larissa Brentano Capeletti, Luciane França de Oliveira, Kaliandra de Almeida Gonçalves, Jessica Fernanda Affonso de Oliveira, Ângela Saito, Jörg Kobarg, João Henrique Zimnoch dos Santos, and Mateus Borba Cardoso. “Tailored Silica–Antibiotic Nanoparticles: Overcoming Bacterial Resistance with Low Cytotoxicity”, Langmuir, 2014, 30 (25), pp 7456–7464. DOI: 10.1021/la4046435
These results reveal that it is possible to tune the emission color of these compounds by changing the chemical environment of the $SbO_4$ matrix.
Researchers at LNLS selected silver nanoparticles of different sizes and evaluated the effect of these nanoparticles on different bacterial strains.