CONTACT & STAFF
For more information on this beamline, contact us.
The SAXS1 beamline is an experimental station dedicated to Small Angle X-ray Scattering (SAXS), operating at a fixed energy of 8 keV. It focuses on structural investigations of materials and biological samples, from the nanometer to a hundred nanometer scale, with applications in material science, chemistry, gels, rheology, structural biology, environmental and geosciences. Other experimental technique available includes Wide Angle X-ray Scattering (WAXS).
Due to the high photon flux, time-resolved SAXS can be performed reaching sub-second time-resolution for kinetic studies.
Several sample environment are made available to the user community, such as (1) furnaces (Linkam THMS600*) allowing temperatures from -200°C up to 600°C, (2) stretching devices (Linkam TST350), (3) circulating solution system with a peristaltic pump for in situ studies, (4) stopped-flow device (Biologic*), (5) autosampler (Spark Holland) for automatic loading of protein solutions.
(*) Multi-user equipments funded by FAPESP: Project 04/09447-9. PROEM – INSTRUMENTACAO DA LINHA SAXS2 DO LNLS: APLICACOES DA TECNICA DE SAXS AO ESTUDO DE MATERIAIS NANOESTRUTURADOS, POLIMEROS DENSOS E SISTEMAS BIOLÓGICOS
For more information on this beamline, contact us.
The following experimental techniques and setups are available to users in this beamline. To learn more about the techniques’ limitations and requirements (sample, environment, etc.) contact the beamline coordinator before submitting your proposal.
| Element | Type | Position [m] | Description |
|---|---|---|---|
| SOURCE | Bending Magnet | 0.0 | Bending Magnet D01 exit B (15°), 1.67 T, 890 µm x 150 µm at 8 keV |
| S0 | Whitebeam Slits | 7.2 | |
| Mono | Toroidal Side-bounce Monochromator | 8.0 | W/B4C Multilayer (500 double layers) on Si substrate |
| S1 | Slits | 8,5 | – |
| S2 | Slits | 14.0 | – |
| S3 | Slits | 16.0 | – |
| Detector | Pilatus 300K | 17.2 | – |
| Parameter | Value | Condition |
|---|---|---|
| Energy range [keV] | 8 | Si(111) |
| Energy resolution [ΔE/E] | 0.1 | Si(111) |
| Beam size at sample [mm2, FWHM] | 1.5 x 1 | at 8 keV |
| Flux density at sample [ph/s/mm2] | 1010 – 1012 | – |
| Instrument | Type | Model | Manufacturer | Specifications |
|---|---|---|---|---|
| Detector | 2D | Pilatus 300K | 172 µm pixel, 487 x 689 pixels, 200 Hz frame rate | Dectris |
| Detector | 2D | Pilatus 100K | 172 µm pixel, 487 x 195 pixels, 200 Hz frame rate | Dectris |
| Autosampler | Automatic sample loading for biology | – | 96 wells plate | Spark Holland |
| Furnace * | Transmission / Capillary / Mica | DSC600 | Temp. Range: -196°C – 600°C, Max. Temp Rate: 30C/s, Solid and Liquid Samples | Linkam |
| Tensile Stretching Stage | Transmission | TST350 | Temp. Range: -150°C – 600°C, in-air | Linkam |
| PALMI | Transmission / Mica | – | Liquid Samples, Thermal bath temp. control available, 300 µL minimum sample volume. | LNLS in-house development |
| PASMI | Transmission | – | Solid Samples, 7 slots, motorized | LNLS in-house development |
| Capillary Cell | Transmission / Capillary | – | Liquid Samples, Thermal bath temp. control available, 80 µL minimum sample volume. | LNLS in-house development |
| Biologic stopped-flow * | Transmission | SFM400 | – | BioLogic Science Instruments |
All beamline controls are done through EPICS (Experimental Physics and Industrial Control System), running on a PXI from National Instruments. The data acquisition is done using a Red Hat workstation with Python scripts, developed at LNLS with SOL group. CSS (Control System Studio) is used as a graphical interface to display and control the beamline devices.
Users are required to acknowledge the use of LNLS facilities in any paper, conference presentation, thesis and any other published material that uses data obtained in the execution of their proposal.
Soren Skou, Richard E Gillilan and Nozomi Ando. Synchrotron-based small-angle X-ray scattering of proteins in solution. Nature Protocols 9, 1727–1739 (2014). DOI:10.1038/nprot.2014.116
With recent advances in data analysis algorithms, X-ray detectors and synchrotron sources, small-angle X-ray scattering (SAXS) has become much more accessible to the structural biology community. Although limited to ∼10 Å resolution, SAXS can provide a wealth of structural information on biomolecules in solution and is compatible with a wide range of experimental conditions. SAXS is thus an attractive alternative when crystallography is not possible. Moreover, advanced use of SAXS can provide unique insight into biomolecular behavior that can only be observed in solution, such as large conformational changes and transient protein-protein interactions. Unlike crystal diffraction data, however, solution scattering data are subtle in appearance, highly sensitive to sample quality and experimental errors and easily misinterpreted. In addition, synchrotron beamlines that are dedicated to SAXS are often unfamiliar to the nonspecialist. Here we present a series of procedures that can be used for SAXS data collection and basic cross-checks designed to detect and avoid aggregation, concentration effects, radiation damage, buffer mismatch and other common problems. Human serum albumin (HSA) serves as a convenient and easily replicated example of just how subtle these problems can sometimes be, but also of how proper technique can yield pristine data even in problematic cases. Because typical data collection times at a synchrotron are only one to several days, we recommend that the sample purity, homogeneity and solubility be extensively optimized before the experiment.
V. Petoukhov, D. Franke, A. V. Shkumatov, G. Tria, A. G. Kikhney, M. Gajda, C. Gorba, H. D. T. Mertens, P. V. Konarev and D. I. Svergun. New developments in the ATSASprogram package for small-angle scattering data analysis. J. Appl. Cryst. (2012). 45, 342-350.DOI:10.1107/S0021889812007662
New developments in the program package ATSAS (version 2.4) for the processing and analysis of isotropic small-angle X-ray and neutron scattering data are described. They include (i) multiplatform data manipulation and display tools, (ii) programs for automated data processing and calculation of overall parameters, (iii) improved usage of high- and low-resolution models from other structural methods, (iv) new algorithms to build three-dimensional models from weakly interacting oligomeric systems and complexes, and (v) enhanced tools to analyse data from mixtures and flexible systems. The new ATSAS release includes installers for current major platforms (Windows, Linux and Mac OSX) and provides improved indexed user documentation. The web-related developments, including a user discussion forum and a widened online access to run ATSAS programs, are also presented.
Michel H. J. Koch, Patrice Vachette and Dmitri I. Svergun. Small-angle scattering: a view on the properties, structures and structural changes of biological macromolecules in solution, Quarterly Reviews of Biophysics, 36(2), pp. 147–227 (2003). DOI: 10.1017/S0033583503003871
A self-contained presentation of the main concepts and methods for interpretation of X-ray and neutron-scattering patterns of biological macromolecules in solution, including a reminder of the basics of X-ray and neutron scattering and a brief overview of relevant aspects of modern instrumentation, is given. For monodisperse solutions the experimental data yield the scattering intensity of the macromolecules, which depends on the contrast between the solvent and the particles as well as on their shape and internal scattering density fluctuations, and the structure factor, which is related to the interactions between macromolecules. After a brief analysis of the information content of the scattering intensity, the two main approaches for modelling the shape and/or structure of macromolecules and the global minimization schemes used in the calculations are presented. The first approach is based, in its more advanced version, on the spherical harmonics approximation and relies on few parameters, whereas the second one uses bead models with thousands of parameters. Extensions of bead modelling can be used to model domain structure and missing parts in high-resolution structures. Methods for computing the scattering patterns from atomic models including the contribution of the hydration shell are discussed and examples are given, which also illustrate that significant differences sometimes exist between crystal and solution structures. These differences are in some cases explainable in terms of rigid-body motions of parts of the structures. Results of two extensive studies – on ribosomes and on the allosteric protein aspartate transcarbamoylase – illustrate the application of the various methods. The unique bridge between equilibrium structures and thermodynamic or kinetic aspects provided by scattering techniques is illustrated by modelling of intermolecular interactions, including crystallization, based on an analysis of the structure factor and recent time-resolved work on assembly and protein folding.
Martel, P. Liu, T. M. Weiss, M. Niebuhr and H. Tsuruta. An integrated high-throughput data acquisition system for biological solution X-ray scattering studies. J. Synchrotron Rad. (2012). 19, 431-434. DOI: 10.1107/S0909049512008072
A fully automated high-throughput solution X-ray scattering data collection system has been developed for protein structure studies at beamline 4-2 of the Stanford Synchrotron Radiation Lightsource. It is composed of a thin-wall quartz capillary cell, a syringe needle assembly on an XYZ positioning arm for sample delivery, a water-cooled sample rack and a computer-controlled fluid dispenser. It is controlled by a specifically developed software component built into the standard beamline control program Blu-Ice/DCS. The integrated system is intuitive and very simple to use, and enables experimenters to customize data collection strategy in a timely fashion in concert with an automated data processing program. The system also allows spectrophotometric determination of protein concentration for each sample aliquot in the beam via an in situ UV absorption spectrometer. A single set of solution scattering measurements requires a 20-30 µl sample aliquot and takes typically 3.5 min, including an extensive capillary cleaning cycle. Over 98.5% of measurements are valid and free from artefacts commonly caused by air-bubble contamination. The sample changer, which is compact and light, facilitates effortless switching with other sample-handling devices required for other types of non-crystalline X-ray scattering experiments.
Scientific publications produced with data obtained at the facilities of this beamline, and published in journals indexed by the Web of Science, are listed below.
Rodríguez-Negrette, A. C. ;Rodriguez-Batiller, M. J.;García-Londoño, V. A. ;Borroni, V. ;Candal, R. J.;Herrera, M. L.. Effect of sucrose esters on polymorphic behavior and crystallization kinetics of cupuassu fat and its fractions, Journal of the American Oil Chemists Society, v.99, n.1, p.27-41, 2022. DOI:10.1002/aocs.12541
Carvalho, B. G. de;Garcia, B. B. M. ;Malfatti Gasperini, A. A. M.;Han, S. W.;de La Torre, L. G.. Hybrid polymer/lipid vesicle synthesis: Association between cationic liposomes and lipoplexes with chondroitin sulfate, Colloids and Surfaces B-Biointerfaces, v.210, p.112233, 2022. DOI:10.1016/j.colsurfb.2021.112233
Seraphim, T. V.;Nano, N. ;Cheung, Y. W. S. ;Aluksanasuwan, S.;Colleti, C. ;Mao, Y.-Q.;Bhandari, V. ;Young, G. ;Höll, L. ;Phanse, S. ;Gordiyenko, Y. ;Southworth, D. R. ;Robinson, C. V.;Thongboonkerd, V. ;Gava, L. M.;Borges, J. C.;Babu, M. ;Barbosa, L. R. S.;Ramos, C. H. I.;Kukura, P. ;Houry, W. A.. Assembly principles of the human R2TP chaperone complex reveal the presence of R2T and R2P complexes, Structure, v.30, n.1, p. 156-170, 2022. DOI:10.1016/j.str.2021.08.002
Cabral, L. ;Persinoti, G. F.;Paixão, D. A. A.;Martins, M. P. ;Morais, M. A. B.de ;Chinaglia, M.;Domingues, M. N.;Sforça, M. L.;Pirolla, R. A. S. ;Generoso, W. C.;Santos, C. A.;Maciel, L. F. ;Terrapon, N. ;Lombard, V. ;Henrissat, B.;Murakami, M. T.. Gut microbiome of the largest living rodent harbors unprecedented enzymatic systems to degrade plant polysaccharides, Nature Communications, v.13, n.1, p.629, 2022. DOI:10.1038/s41467-022-28310-y
Malheiros, B.;Castro, R. D. de ;Lotierzo, M. C. G. ;Casadei, B. R. ;Mariani, P.;Barbosa, L. R. S.. Influence of hexadecylphosphocholine (Miltefosine) in phytantriol-based cubosomes: A structural investigation, Colloids and Surfaces A-Physicochemical and Engineering Aspects, v.632, p.127720, 2022. DOI:10.1016/j.colsurfa.2021.127720
Brum, L. F. W. ;Santos, C. dos;Santos, J. H. Z. dos;Brandelli, A.. Structured silica materials as innovative delivery systems for the bacteriocin nisin, Food Chemistry, v.366, p.130599, 2022. DOI:10.1016/j.foodchem.2021.130599
Palacio, G. ;Pulcinelli, S. H.;Santilli, C. V.. Fingerprint of semi-crystalline structure memory in the thermal and ionic conduction properties of amorphous ureasil–polyether hybrid solid electrolytes, RSC Advances, v.12, n.9, p.5225-5235, 2022. DOI:10.1039/d1ra09138g

Português:
Cabana experimental da Linha de luz SAXS1.
English:
SAXS1 beamline experimental hutch.
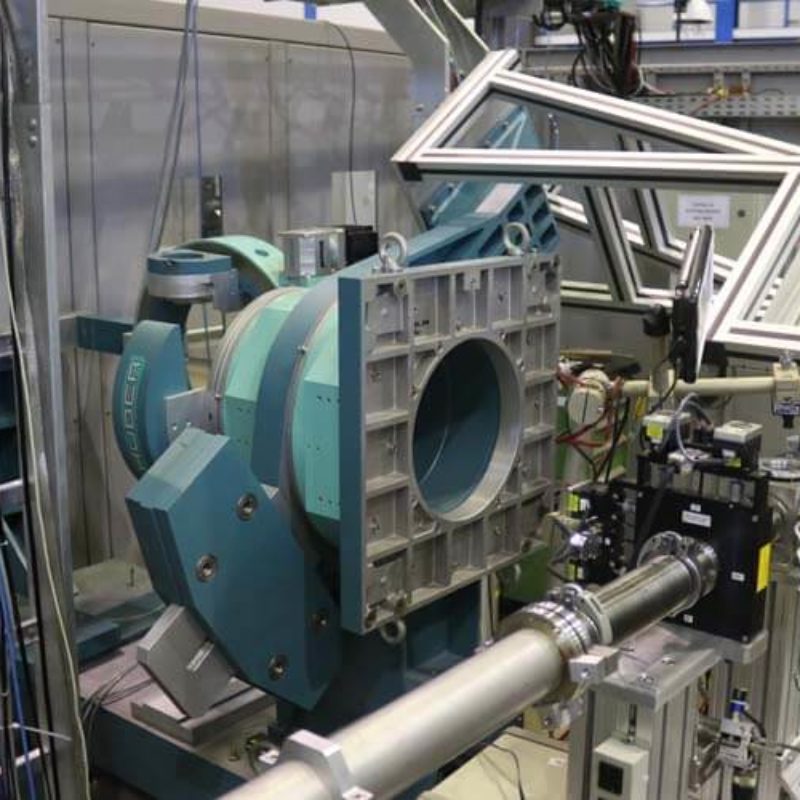
Português:
Goniômetro usado como suporte de detector, para realização de WAXS.
English:
Goniometer used for detector support, for the pourpose of doing WAXS.

Português:
Câmara de porta-amostra, com controle de temperatura por banho térmico e visualização da amostra por câmera.
English:
Sample holder chamber, with thermal bath temperature control and sample visualized by camera.
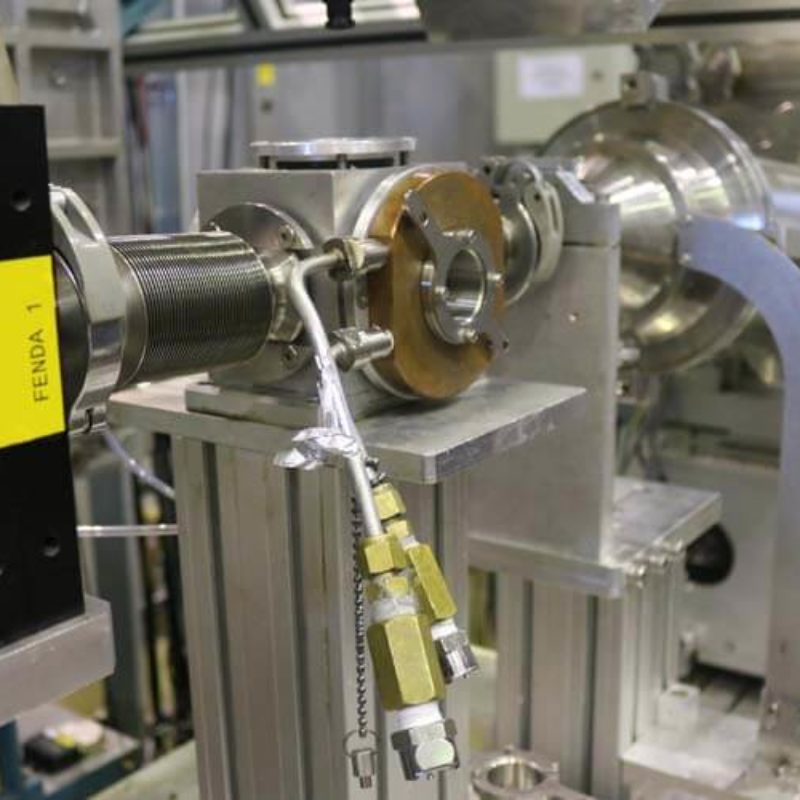
Português:
Vista lateral do porta-amostra.
English:
Lateral view of the sample holder.

Português:
Detector Pilatus 300K da Dectris, usado para aquisição de imagens de SAXS.
English:
Pilatus 300K detector, from Dectris, for acquisition of SAXS images.

Português:
Detector Pilatus 100K da Dectris, usado para aquisição de imagens de WAXS.
English:
Pilatus 100K detector, from Dectris, for acquisition of WAXS images.

Português:
Porta-amostra TST350, da Linkam, para estudo de amostras sob tensão mecânica, com controle de temperatura (-196°C - 350°C).
English:
TST350 sample holder, from Linkam, for studies of samples under mechanical tensile, with temperature control (-196°C - 350°C).

Português:
Porta-amostra DSC600, da Linkam, para estudo de amostras com controle de temperatura (-196°C - 600°C).
English:
DSC600 sample holder, from Linkam, for studies of samples with temperature control (-196°C - 600°C).
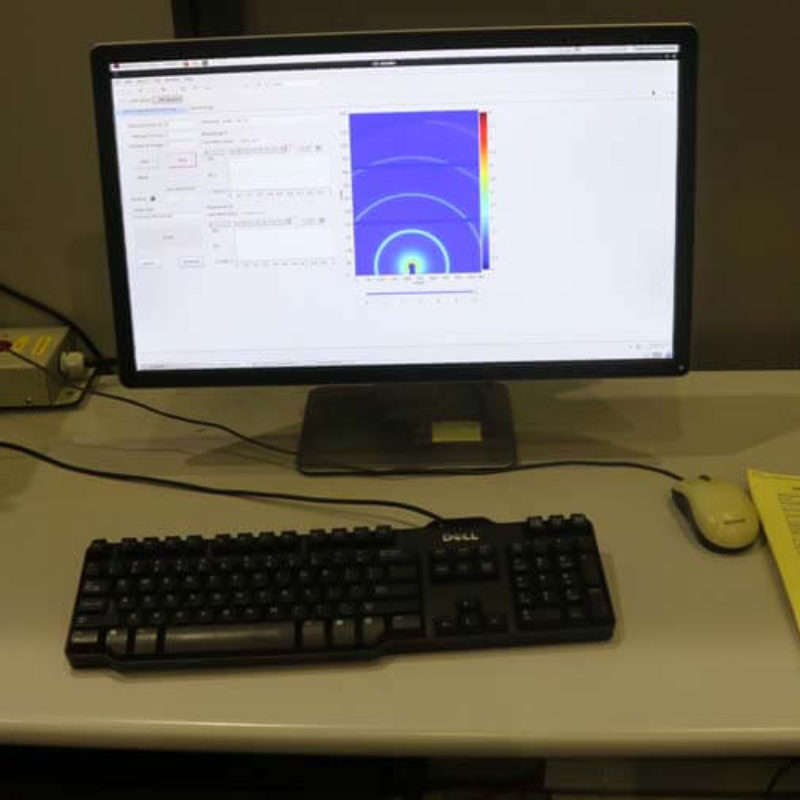
Português:
Programa de controle para realização dos experimentos de SAXS e WAXS.
English:
Control software for doing SAXS and WAXS experiments.

Português:
Diferentes tipos de porta-amostras personalizados da linha de luz SAXS1, para diversos tipos de amostras, como sólidos, líquidos e géis.
English:
Different type of sample holder developed in-house of SAXS1 beamline, for several types of samples, as solids, liquids and gels.

Português:
Estação de controle e preparo de amostra da linha de luz SAXS1.
English:
Control station and sample preparation of SAXS1 beamline.