
An example of nanocrystals are quantum dots, which are capable of emitting light in well-defined wavelength distributions. (Photo: Alexei Antipov)
Research applies unprecedented technique in Brazil for the investigation of crystalline nanoparticles
The development of faster and more efficient electronic devices, better catalysts for the chemical industry, alternative energy sources, and so many other technologies depends increasingly on the in-depth understanding of the behavior of materials at the nanometer scale. The properties of particles on this scale may be completely different from the properties of the same material in its macroscopic version. In addition, nanoparticles of different sizes and shapes can have completely different characteristics, even though they are formed by the same material.
The possibility of regulating the optical and electrical properties of nanoparticles by controlling their composition, shapes and sizes opens the door to an immense variety of applications. In this context, nanocrystals – nanometric particles that have a crystalline structure – have attracted great interest.
A crystal is a type of solid whose atoms or molecules are arranged in a well-defined three-dimensional pattern that repeats itself in space on a regular basis. The optical and electrical properties of crystalline materials depend not only on the atoms or molecules that constitute them but also on the way they are distributed. Therefore, defects or impurities present during crystal formation cause a disorder in the crystal structure. The consequent modification in the electronic structure of the crystal can lead to changes in its properties.
Thus, when modifications in the crystalline structure are induced in a controlled manner, it is possible to control and optimize the properties of materials for specific applications. This is the case, for example, of the addition of dopants to the structure of silicon crystals in the electronics industry.
Nanocrystals also have their properties influenced by the presence of defects and impurities. However, there is one more factor to consider: during its synthesis, nanocrystals have the tendency to aggregate into larger nanoparticles. For this to be avoided, organic molecules are added to the synthesis. These ligands distribute themselves around the surface of the nanoparticles stabilizing their growth. The presence of a ligand may, however, modify the crystalline structure, affecting the properties of the particle. Thus, it is essential to understand the interactions between ligand and crystal, as well as the ligand exchange process, and its effect on the structural disorder.
In this context, researchers from the Brazilian Center for Research in Energy and Materials (CNPEM) and collaborators used the facilities of the Brazilian Nanotechnology National Laboratory (LNNano) and the Brazilian Synchrotron Light Laboratory (LNLS) to elucidate the effect of ligand exchange on zirconia $\rm ZrO_2$ nanocrystals and the consequent changes in structural disorder [1].
The researchers used different advanced characterization techniques – extended X-ray absorption fine structure (EXAFS), pair distribution function (PDF) and high-resolution transmission electron microscopy (HRTEM) – combined with first principles theoretical calculations based on the density functional theory (DFT) to reveal the structure of the nanocrystals.
Pair distribution function (PDF) analysis, also called total scattering analysis, is an experimental technique that provides structural information on amorphous, non-crystalline, nanocrystalline or nanostructured materials. This type of analysis is usually performed in neutron sources or in synchrotron light sources, where it benefits from high-brightness, high-energy X-rays. However, LNLS’ current synchrotron light source, called UVX, does not produce X-rays suitable for this kind of experiment.
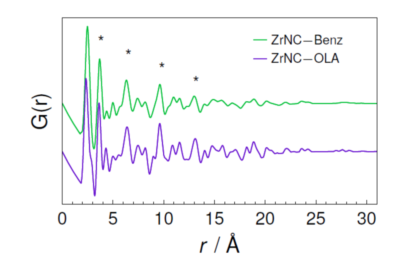
PDF analysis obtained from electron diffraction data for nanocrystals before (ZrNC-Benz) and after ligand exchange (ZrNC-OLA). Reprinted with permission from J. Phys. Chem. Lett. 2019, 10, 7, 1471-1476. Copyright 2019 American Chemical Society.
To circumvent this limitation, the researchers used diffractograms obtained by tunneling electron microscopy in LNNano for the PDF analysis. When compared to the conventional technique, PDF analysis using electron diffraction has the advantage of using micrograms of material and a very low data collection time.
The researchers argue that the structural disorder identified by EXAFS and PDFs arises from the competition between the contraction tendency of the $\rm ZrO_2$ nanocrystals bond length and the presence of ligands that decrease this strain of the chemical bonds. Therefore, the ligand exchange process causes a decrease in the structural disorder of the nanocrystals, but the specific ligand has a weak influence on the decrease of structural disorder.
These results have a direct impact on the development of functional nanomaterials, especially for those where surface-localized structural disorder plays an important role in mechanical, photoluminescence, electronic transport and catalytic properties.
The mastery over the electron diffraction PDF technique and its availability at the open facilities of LNNano open new possibilities for research in Brazil. In addition, Sirius, the new Brazilian synchrotron light source, will have the appropriate parameters to perform several advanced experiments, such as the PDF analysis.
Source: [1] Gabriel R. Schleder, Gustavo M. Azevedo, Içamira C. Nogueira, Querem H. F. Rebelo, Jefferson Bettini, Adalberto Fazzio, and Edson R. Leite, Decreasing Nanocrystal Structural Disorder by Ligand Exchange: An Experimental and Theoretical Analysis, The Journal of Physical Chemistry Letters 2019 10 (7), 1471-1476. DOI: 10.1021/acs.jpclett.9b00439
Research evaluates combination of graphene and hexagonal boron nitride for opto-electronic devices of the future
Research develops nanostructured material with high oxygen storage and release capacity for the improvement of catalytic converters