The studies of the size, structure and magnetic properties of the multifunctional brick-like $ \rm Ag@Fe_3O_4$ NPs obtained reveal them as possible candidates for advanced medical purposes.
Nanotechnology presents a very fast growth owing to a vast number of applications. In medicine, for example, it is possible to envisage a strong improvement in the efficiency of the magnetic resonance imaging or in the development of non-conventional diagnostics or therapies. The pace of development of the area is strongly dependent on the improvement of synthesis routes, which would allow producing, in a controlled way, new materials capable to act in the intracellular environment.
Silver nanoparticles (NPs) have been intensively studied due to their highly effective bactericide properties but they present a lack of biocompatibility. The antibacterial mechanism is associated to the release of silver ions.
Recently, M. E. F. Brollo et al. (1) described a temperature-paused single-step synthetic route that they developed to produce novel $ \rm Ag@Fe_3O_4$ compact core-shell nanostructures. These nanoparticles are formed by a silver nucleus wrapped by a compact magnetite shell. Owing to the curious rectangular shape, they denote these particles as brick-like nanoparticles (BLNs). For medical applications, an $ \rm Ag@Fe_3O_4$ core-shell structure allows one to add a magnetic functionality to silver properties. Such nanostructure could lead to interesting advances to solve the lack of biocompatibility of silver, eliminating its contact with tissues (iron oxide can be considered biocompatible, at least up to the mg/ml range).
In order to help the understanding of the structure formation, the morphology and crystalline structure were studied by means of transmission electron microscopy (TEM), X-ray diffraction (XRD) and X-ray absorption spectroscopies: X-ray absorption near edge spectra (XANES) and extended X-ray absorption fine structure (EXAFS). The magnetic characterization was performed by conventional SQUID magnetometry.
Synthesis
Usually, the so-called $ \rm Ag-Fe_3O_4$ core-shell NPs reported in the scientific literature are, in fact, combined NPs which form dimer or flower-like combinations. They are synthesized using a conventional two-steps protocol in which an iron oxide is produced by thermal decomposition on previously synthesized Ag seeds. The authors of this work developed a novel single-step protocol, in order to reduce manipulation between the steps and facilitate the synthesis control. In this case, the Ag seeds are formed in the same reaction mixture, just before the iron oxide formation.
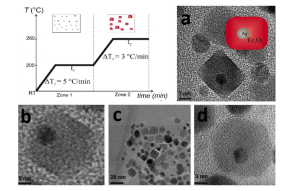
Figure 1. Temperature profile of the temperature-paused single-step thermal decomposition synthesis. Boxes sketch the expected predominant structures for each time zone. Typically, both waiting times are 120 minutes. Images: TEM images of BLNs obtained following the temperature-paused single-step protocol. Ag corresponds to the dark contrast, while lighter particles correspond to magnetite. Plain magnetite nanoparticles, which are formed are also shown in c). a) b) and d) are different amplifications of BLNs in order to understand the structure.
The thermal decomposition of the $ \rm Fe_3O_4$ precursor occurs on a silver colloid formed in the same reaction mixture by the addition of $ \rm AgNO_3$ salt. The iron precursor in the form of Fe (III) acetylacetonate complex (3 mmol), a diol reduction agent 1, 2-hexadecanediol (1 mmol), a mixture of surfactants composed by oleylamine and oleic acid (12:3 mmol) and a silver salt $ \rm AgNO_3$ (1 mmol) were added on benzyl-ether (boiling point of 298°C) at room temperature on a three-neck round-bottom flask mounted on a temperature-controlled reflux system. The reaction mixture was magnetically stirred in Ar inert atmosphere without vacuum application. The process is shown in Figure 1.
The temperature is increased in a controlled way. Usually, in the conventional two-steps protocol, a temperature pause is introduced before the reflux temperature to ensure the reagent solution and proper homogeneity. The pause introduced in the present single-step protocol has a different purpose: it is done at 200°C, in order to separate the growth of silver (zone 1) from the proper thermal decomposition of the iron precursor (zone 2). Thus, the role of the temperature pause is essentially to divide the Ag and the iron oxide production inside a single reaction, avoiding the necessity of manipulation and the time consuming gap between steps. If the protocol is performed without the pause, it results in the well-known non-homogeneous core shell NPs provided vacuum is applied. With the pause the results are completely different.
Morphology and Brick-like Structure
Figure 1 shows TEM images of the particles obtained by the single-step reaction. They were measured on the 200 keV JEOL-JEM 2100 microscope at the Brazilian Nanotechnology National Laboratory (LNNano). On the images, Ag is in dark contrast, while lighter structures correspond to magnetite. Only two types of structures were observed (values in volume fractions): 85.5% of BLNs and 14.5% of plain $ \rm Fe_3O_4$ nanoparticles. Within each single BLN, approximately 99% of the volume corresponds to the magnetite shell, while just 1% to the silver core volume. The amount of conventional structures such as flower-like or dimer nanoparticles was negligible, in fact, less than 1%. It is worth noticing that the magnetite shell presents an unexpected parallelepiped geometry with average base of 13(2) nm and 15(3) nm (in case of morphological parameters, the parenthesis that represents the error in the last digit corresponds to the half width at half maximum, HWHM, extracted from the size distribution curve after counting around 40 nanoparticles). This ‘‘brick-like’’ structure differs from conventionally produced core-shell structures. The plain magnetite nanoparticles display a nearly spherical shape with diameter of 7.0(5) nm. As seen in Figure 1 d) few isolated, nearly- spherical core-shell nanoparticles with diameter 13(3) nm were also observed. Figure 2 b) shows the size distribution of the silver core in the BLNs. The log-normal fit leads to a mean diameter of 4.7(1) nm.
Before the pause, the partial oxidation of Fe (III) to Fe (II) is visible at 70°C when the solution color changes from red to black. The Ag reduction occurs in this interval and during the pause. In this pause, introduced at 200°C, the iron-oleic complex, which is the intermediary product and the proper iron decomposition precursor, is formed through ligand exchange from the acetyl-acetonate complex. The oleic acid is the ligand with the highest iron coordination capacity and a time (t1) of 2 h is reported to be enough to produce the quantitative ligand exchange. In this temperature, there is no proper decomposition to iron oxide, even in a seed-mediated reaction, i.e., in a heterogeneous medium, as in this case. It seems to indicate that parallel to ligand exchange in the iron precursor, the action of surfactants (usually not present in the two-steps protocol) on the Ag seeds in this pause play a key role as steric stabilizing agent restricting the seed size. The sizes in the silver core are consistent with a surfactant-mediated limitation to a second coalescence in a silver growth model proposed in the reference (2). The absence of a bimodal size distribution and polycrystalline structures also seems to corroborate this idea. We have observed that pauses below 200°C reduce the quantity of BLNs. The highest temperature to perform the pause is given by the magnetite nucleation temperature between 210 and 250°C. After the pause, the iron complex decomposes and a mixed valence iron oxide nucleates. Finally, during the ramp and the reflux time (t2) of 2 hours, growth and ripening processes occurs. The final reaction mixture exhibits a surface metallic blue color due to the presence of silver particles.
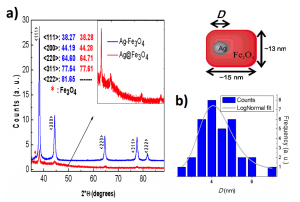
Figure 2. (a) Conventional dimer $ \rm Ag-Fe_3O_4$ (blue) and BLN (red) X-ray diffraction patterns. Inset: enlargement of the X-ray diffraction pattern of the BLN sample schemed. (b) Size distribution of the Ag core within BLNs. The data was obtained from 34 counts of the TEM image analysis. The gray line corresponds to a log-normal fit.
Figure 2 shows X-ray diffraction patterns of the crystallographic silver planes of BLNs and a sample of conventional $ \rm Ag-Fe_3O_4$ in dimer form. Both spectra agree qualitatively. In the case of $ \rm Ag-Fe_3O_4$ dimer, the peaks are sharper than those of BLN. This is probably due to a lower crystallographic ordering in a smaller Ag particle. The small shifts observed in the position of the Ag plane peaks are probably related to interface effects between the silver and the magnetite. In the BLNs, the interface area is larger, corresponding approximately to 4% of the total silver atoms. Also, if one considers the difference in sizes, the lattice constant reduction in silver could become non-negligible.
XANES and EXAFS: Oxidation State
The oxidation state was studied through X-ray absorption near edge spectra (XANES) and extended X-ray absorption fine structure (EXAFS), done at the wiggler XDS beamline of the Brazilian Synchrotron Light Laboratory (LNLS). A sample of plain $ \rm Fe_3O_4$ NPs (hereafter named as P) with well-defined cubic geometry (edges of 27(4) nm) was chosen for comparison because of its closer magnetic properties. XANES features at the Fe K-edge mainly resemble those corresponding to magnetite $ \rm Fe_3O_4$. The pre-edge energy position is compatible with a Fe (III)-Fe (II) mixture. The main component of the pre-edge peaks of $ \rm Fe_3O_4$ arises from tetrahedral Fe as it is observed in the first peak for both samples. The shoulder corresponds to octahedral Fe (III)–Fe (II) ions. At lower energies, the characteristic low intensity peak corresponds to hexa-coordinated Fe (II). In BLN sample, the shoulder is shifted to high energies, higher than 1.1 eV. This limb is expected in nanometric samples that contain a maghemite fraction. The average iron oxidation state was 2.62(1) for P sample and 2.73(2) for BLN sample. The value expected for the $ \rm Fe_3O_4$ magnetite phase is 2.67, which reveals that most of the iron oxide phase in BLN is indeed magnetite. A small amount of maghemite phase was also observed, as expected considering the presence of a small fraction of plain nanometric magnetite (which is absent in the P sample), where the surface oxidation becomes more relevant. The peak position, which is sensitive to oxidation state, is slightly shifted to higher energies for the BLN as expected for more oxidized species. The decrease in the pre-edge peak intensity reveals that the iron environment for BLN is more centrosymmetric than in P.
From the EXAFS data, it is clear that sample P presents a closer similarity to Fe3O4 bulk than BLN sample. The BLN sample is closer to bulk $ \rm Fe_2O_3$ for the first and second environments shells. This similarity between BLN and bulk $ \rm Fe_2O_3$ on the EXAFS measurement and the existence of small amount of maghemite phase obtained from XANES could be ascribed to the surface oxidation in the BLN and the non-negligible amount of plain magnetite nanoparticles in the sample.
Magnetization
The magnetization characterization was performed on dried powder samples using a commercial Quantum Design SQUID magnetometer. DC magnetic properties were measured on the BLN sample and compared with sample P, which corresponds to plain magnetite produced without introducing silver seeds. Although the size of P is bigger than the magnetic component of the BLN, it exhibits closer magnetic properties; namely, a similar ZFC-FC behavior, saturation magnetization and coercive field. The hysteresis loops for both samples at 300 K show that the particles are close to the superparamagnetic regime, showing rather low coercive fields: 1.8(3) 3 103 A/m and 2.4(3) 3 103 A/ m, for BLN and P, respectively.
Conclusion
The studies of the size, structure and magnetic properties of the multifunctional brick-like $ \rm Ag@Fe_3O_4$ NPs obtained reveal them as possible candidates for advanced medical purposes. Heating efficiency studies for hyperthermia purposes are in perspective to be done. Reaction kinetics in order to understand the growing mechanisms and the subsequent synthesis control will also be studied in detail in future due to the restriction in the silver core size.
Sources:
(1) Maria Eugênia F. Brollo, Román López-Ruiz, Diego Muraca, Santiago J. A. Figueroa, Kleber R. Pirota & Marcelo Knobel, “Compact Ag@Fe3O4Core-shell Nanoparticles by Means of Single-step Thermal Decomposition Reaction”, Scientific Reports 4, 6839 (2014). DOI:10.1038/srep06839
(2) T. K. Thanh, N. Maclean& S. Mahiddine. Chem. Rev. 114, 7610, 2014
The authors have demonstrated the effective biological activity of a noncomplex nanomaterial against susceptible and antibiotic-resistant bacteria without significant cytotoxicity.
These results reveal that it is possible to tune the emission color of these compounds by changing the chemical environment of the $SbO_4$ matrix.