Enzyme extracted from microorganisms from Amazonian lake shows potential for biofuel production
The growing understanding that the rise in average temperature of the planet is caused by human action has, in recent years, intensified the search for clean, renewable and cheap energy sources. One alternative source are the second generation biofuels produced from agricultural waste, such as sugar cane straw and bagasse, which are mainly composed of cellulose. The production of second generation ethanol, for example, breaks down this cellulose into simpler sugars with the use of several enzymes, followed by fermentation into ethanol.
Among the enzymes required for this process, the so-called endo- and exoglucanases act directly on cellulose producing cellobiose, a disaccharide composed of two glucose molecules. Next, cellobiose needs to be broken down into glucose by $\rm \beta$-glucosidase enzymes. However, these substances have their action inhibited by the presence of their own products. There is thus an increasing demand for new enzymes that not only resist inhibition, but also have optimum activity in the temperature and pH ranges suitable for industrial production.
The Amazon region is home to the largest hydrographic basin and to one of the largest biodiversities in the world. Research into this wide variety of organisms may lead to the discovery of various substances with biotechnological applications. For example, microorganisms present in rivers and lakes of the region produce enzymes which decompose plant matter and can be useful in the production of second generation biofuels.
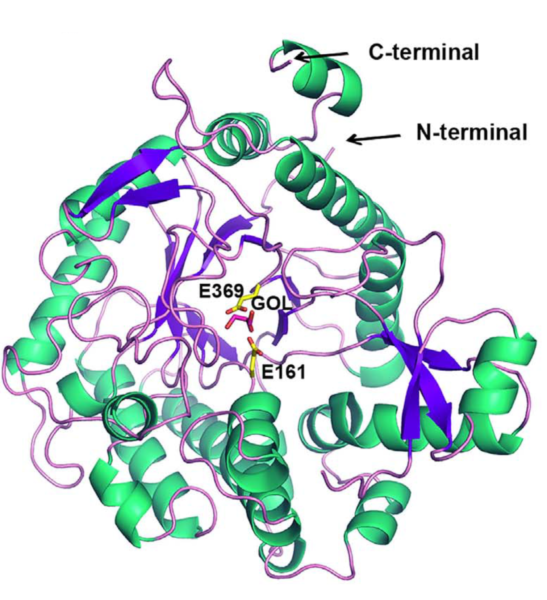
Overall structure of AmBgl-LP monomer with the catalytic residues represented as sticks (carbon atoms in yellow).
In a recent study [1], Brazilian researchers identified a new $\rm \beta$-glucosidase enzyme, from the analysis of microorganisms present in the Lake Poraquê, adjacent to the Solimões River. The MX2 and SAXS1 beamlines of the Brazilian Synchrotron Light Laboratory (LNLS) were used in the structural characterization of the enzyme, called AmBgl-LP, which was shown to be similar to other $\rm \beta$-glucosidase of the GH1 family. However, differently from the enzymes of this group, AmBgl-LP dimerizes (combines in pairs) when in solution.
The group also verified that the AmBgl-LP has characteristics which are compatible with the process of Simultaneous Saccharification and Fermentation (SSF). In this important process for the reduction of costs in the industry, enzymatic cocktails break the cellulose into glucose simultaneously with the action of yeast in fermenting the sugars into ethanol.
This is the first time that an enzyme from an Amazonian lake sample is isolated, produced and characterized by metagenomics, which is the study of genetic material obtained directly from environmental samples containing a wide variety of organisms. According to the researchers, this approach not only proved to be valuable for the search of new enzymes for the degradation of cellulose in industrial processes, but also contributes to the understanding of the structural diversity of the GH1 enzyme family.
This research was the result of collaboration between researchers from the Brazilian National Biosciences Laboratory (LNBio) and the Brazilian Bioethanol Science and Technology Laboratory (CTBE) – both part of the Brazilian Center for Research in Energy and Materials (CNPEM) – and researchers from the University of São Paulo, Federal University of São Carlos and the company Petrobras. In addition to the LNLS beamlines, LNBio’s open facilities were also used.
Source: [1] Danyelle Toyama, Mariana Abrahão Bueno de Morais, Felipe Cardoso Ramos, Letícia Maria Zanphorlin, Celisa Caldana Costa Tonoli, Augusto Furio Balula, Fernando Pellon de Miranda, Vitor Medeiros Almeida, Sandro Roberto Marana, Roberto Ruller, Mario Tyago Murakami, Flavio Henrique-Silva, A novel β-glucosidase isolated from the microbial metagenome of Lake Poraquê (Amazon, Brazil), Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics, 2018. DOI: 10.1016/j.bbapap.2018.02.001
Research investigates mechanism of absorption and transport of zinc nanoparticles
Research analyzes the deactivation of nickel catalysts in dry reforming of methane