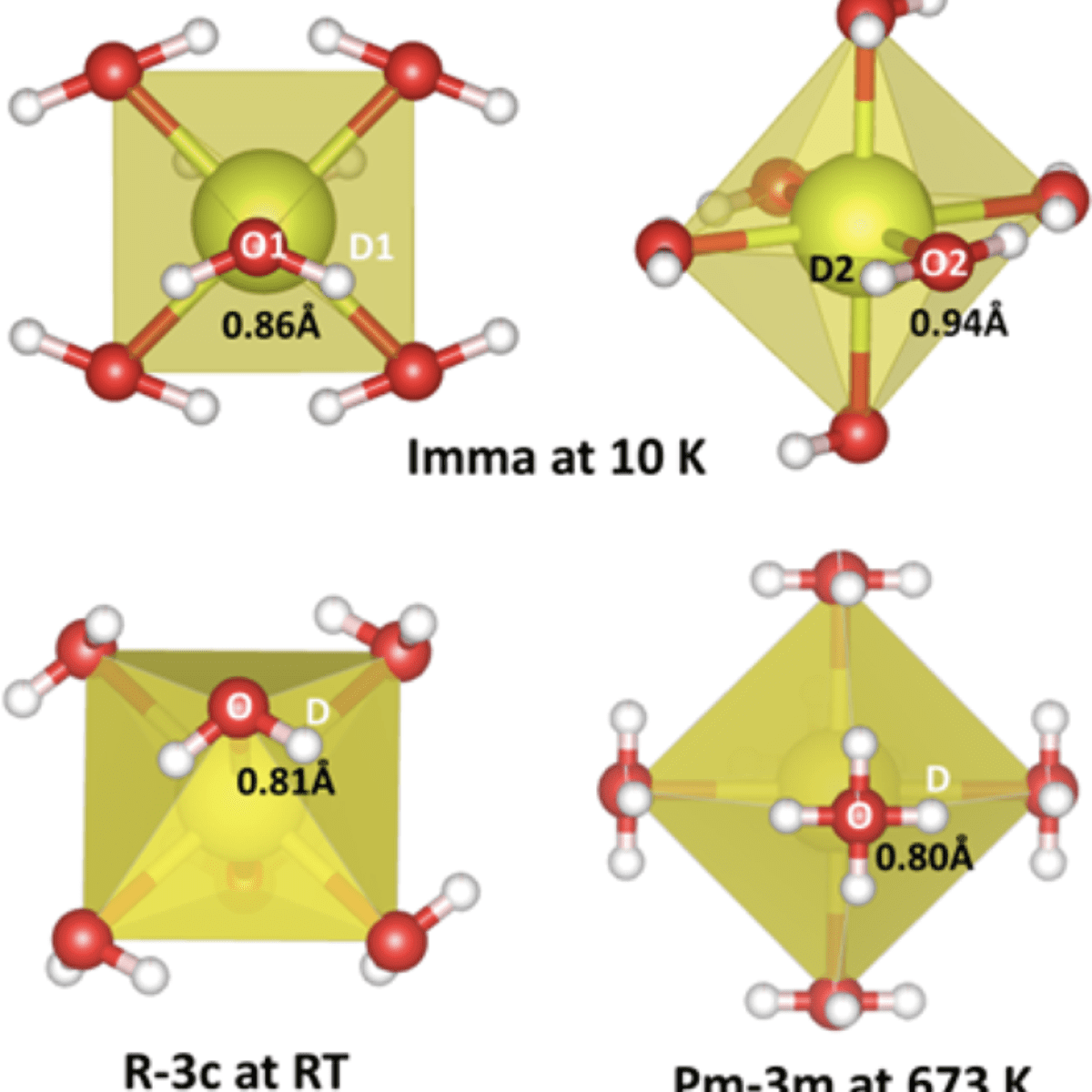
A detailed analysis highlights several structural complexities of the (BZCY72) perovskite.
High-temperature electrochemical devices exhibiting the preferential transfer of protons have the potential to produce a shift to a sustainable energy economy in which hydrogen replaces hydrocarbon sources as the principal fuel for stationary power and transportation. Protonic ceramic fuel cells (PCFCs), with a proton-conducting ceramic electrolyte membrane, cleanly convert the chemical energy of hydrogen to electrical energy in an intermediate temperature range (773−1023 K).
This interval neatly alleviates the technological problems and costs associated with the higher operating temperatures of contemporary solid oxide fuel cells based on an oxide-ion conducting electrolyte and those of the polymeric devices which require expensive electrocatalysts due to the low operation temperature, below 373 K.
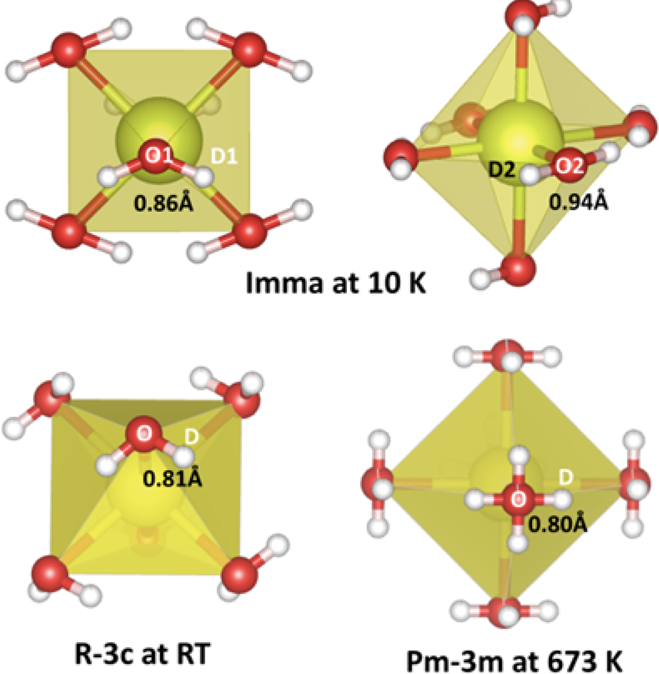
The best proton-transport characteristics are observed in perovskite oxides ($ \rm ABO_{3}$) with large basic A cation (e.g., Ba, Sr) and large tetravalent B cations (e.g., Zr, Ce), which are doped with a lower valence cation on the B site to form oxygen vacancies in the dry state. In water-containing atmospheres, the oxygen vacancies are replaced by hydroxyl groups with the second proton of the water molecule attaching to another lattice oxygen. The materials of choice for PCFCs, hydrogen separators, and membrane reactors increasingly center on $ \rm Ba(Ce,Zr)O_{3-\delta}$–based solid solutions for a compromise between moderately high proton conductivity and good stability, respectively offered by the $ \rm BaCeO_{3}$ and $ \rm BaZrO_{3}$ end-members. Much recent work adopts $ \rm BaZr_{0.7}Ce_{0.2}Y_{0.1}O_{3-\delta}$ (often referred to in the literature as BCZY27) as the optimized composition as development toward commercialization of proton-conducting membranes continues. A full structural analysis is thus required for further understanding and development of this technologically important material because perovskites very often exhibit a number of symmetry changes with pressure, temperature, or composition associated with tilting of the $ BO_{6}$ octahedra, with the adopted tilt system very much guided by the tolerance factor.
Recently Glenn C. Mather et al., [1] have employed high-resolution neutron diffraction, complemented by synchrotron radiation XRD data (SR-XRD), to perform a structural analysis of BZCY72 in the temperature range 10− 1173 K, involving space-group determination and phase transitions with temperature, investigation of chemical expansion resulting from hydration, and location of deuteron sites. The assignment of space group relies on the detection of supercell reflections attributable to subtle movements of the oxygen positions arising from octahedral tilting which are largely imperceptible to X-rays due to the low X-ray scattering length of oxygen. In contrast, neutron diffraction is much more suited to determine symmetry changes as the neutron scattering length of oxygen is comparable with those of the cation species. Deuterated samples were used in this study to avoid the large incoherent scattering cross-section of protons to thermal neutrons and improve the likelihood of locating deuterons within the oxide lattice. Neutron powder diffraction (NPD) data were collected at very low temperature (10 K) to freeze out the influence of phonon interactions and reduce thermal factors.
Experiment
$ \rm BaZr_{0.7}Ce_{0.2}Y_{0.1}O_{3-\delta}$ (BZCY72) was made of powder pellets. The pellets were then crushed and milled in an agate mortar and pestle in acetone. Deuterated material for NPD was prepared on heating the as-prepared powder to 1173 at 5 $ \rm K.min^{-1}$ in air bubbled through $ \rm D_{2}O$ at room temperature (RT), followed by a 2h dwell at this temperature then cooling to RT at 1 $ \rm K.min^{-1}$. Phase purity was confirmed by powder XRD measurements. NPD measurements were performed on powders housed in vanadium cans at the high-resolution D2B diffractometer at the Institut Laue Langevin (Grenoble, France). Monochromatic beam wavelengths of 1.05 and 1.594 Å were used.
Synchrotron radiation X-ray diffraction (SR-XRD) data were recorded at the D10B-XRD1 beamline of the Brazilian Synchrotron Light Laboratory (LNLS, Campinas, Brazil) on an as-prepared (undeuterated) sample mounted on a ceramic sample holder and heated in the range 298−1173K. The X-ray wavelength was set at 1.54915Å, and data were collected in the range 20≤2Θ≤90° with a step length of 0.005° and a step counting time of 2s. The sample was heated at a rate of 10 $ \rm K.min^{-1}$, and a dwell time of 10 min was employed at each temperature before performing the SR-XRD scan.
Results
The neutron data allowed to measure the structural parameters, selected interatomic distances at 10 K, RT and 673K and to obtain the space group (Imma at 10 K, R3̅c at RT and Pm3̅m at 673 K). A volume discontinuity on heating indicates a first-order phase transition from orthorhombic (Imma) to rhombohedral symmetry (R3̅c) between 85 and 150 K (Fig. 1). A further transition to cubic symmetry (Pm3̅m,) takes place at ~570 K: from the temperature dependence of the octahedral tilt angle it is a second order transition. The stability field of the cubic phase was extended on cooling in the dehydrated state to 85 K. Expansion/contraction of the unit-cell volume on heating in low vacuum and air, respectively observed by neutron diffraction and synchrotron X-ray diffraction, was described with a point-defect model involving the temperature dependence of the water content and thermal expansion. Isotropic strain in the hydrated state is apparent on analysis of the broadening of the neutron-diffraction reflections during heating and cooling cycles.
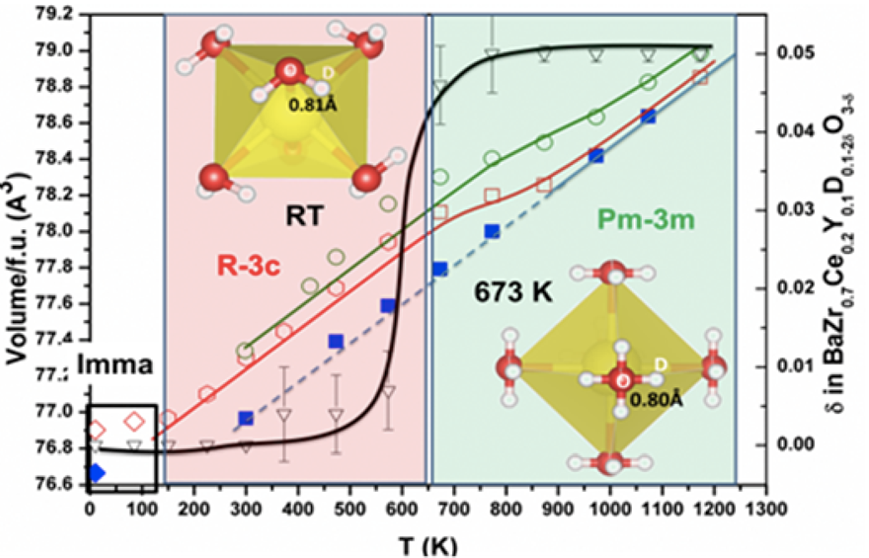
Figure 1. Experimental (symbols) and calculated (colored lines) pseudocubic lattice constant of BZCY72 determined from neutron diffraction data on heating (open red symbols) and cooling (blue closed symbols) in low vacuum and synchrotron X-ray diffraction data (open green symbols) on heating in air.
The calculated data employs a model based on thermal expansion and chemical expansion due to hydration; $ \rm pH_{2}O$ values of $ 1 \times 10^{-3}$ and $ 2 \times 10^{-2}$ atm were adopted for NPD and SR-XRD experiments, respectively. The oxygen loss occurring from the nominal formula of the hydrated material on heating as determined from refinement of the oxygen occupancy of the NPD data is also shown with reference to the secondary y-axis (inverted black triangles, with black line shown as guide for the eye). Schematic diagrams of the deuterium position (white spheres) in relation to oxygen (red spheres) and the $ \rm BO_{6}$ octahedra (yellow) for RT and 673 K are also shown.
Conclusion
The $ \rm BaZr_{0.7}Ce_{0.2}Y_{0.1}O_{3-\delta}$ (BZCY72) perovskite is emerging as of the strongest candidates for a number of applications involving high-temperature proton transport, including protonic ceramic fuel cells, protonic ceramic electrolyzer cells, and reactor Ce0membranes for processes such as methane dehydroaromatization.
A detailed analysis by high resolution neutron diffraction and synchrotron X-ray diffraction highlights several structural complexities of this technologically important phase. As is the case for similar proton-conducting perovskites, the phase stability is highly dependent on the degree of hydration. A transition from orthorhombic to rhombohedral symmetry occurs above 85 K on heating and then from rhombohedral to cubic symmetry at ∼ 570 K for the hydrated material (diffracting medium, low vacuum), but the latter transition occurs below RT on cooling in the dehydrated state. The hydrated condition, which is preserved above the phase-transition temperature, is associated with chemical cell expansion and strain broadening of the neutron-diffraction peaks, with cell contraction and alleviation of strain taking place on heating to dehydration.
The two deuteron sites of a deuterated sample at 10 K (space group, Imma ) are similar to those of chemically similar perovskites, lying close to a plane bisecting the O− O octahedral edge at a distance of ∼ 0.90 Å from the bonding oxygen and ∼ 2 Å from the neighboring B-cation sites.
Source: [1] Glenn C. Mather, Gemma Heras-Juaristi, Clemens Ritter, Rodolfo O. Fuentes, Adilson L. Chinelatto, Domingo Pérez-Coll, and Ulises Amador, Phase Transitions, Chemical Expansion, and Deuteron Sites in the BaZr0.7Ce0.2Y0.1O3−δ Proton Conductor. Chem. Mater., 2016, 28 (12), pp 4292–4299. DOI: 10.1021/acs.chemmater.6b01095
The results demonstrate for the first time the antiviral activity of mSiO2 particles.
The small interface roughness and polarization phenomena lead to a larger charge-carrier mobility and better operational stability.