The results demonstrate for the first time the antiviral activity of mSiO2 particles.
Viruses are among the most harmful pathogens and are responsible for the death of millions of people every year. Even after the development of the highly active anti-retroviral therapy which improved the quality of life and increased the life expectancy of patients with AIDS, human immunodeficiency virus (HIV) alone was responsible for the death of 1.2 million people in 2014. The development of new strategies to fight viruses is necessary, as antiviral therapies and other efficacious vaccines are not available for many viral diseases.
Tailored nanostructures have emerged as a possible alternative, since they have shown activity against different viruses, including hepatitis B, virus human immunodeficiency virus, herpes simplex virus, respiratory syncytial virus, adenovirus, monkey pox virus, influenza, and H1N1 influenza A viruses. However, not much is known about the nanostructures mechanisms of antiviral action.
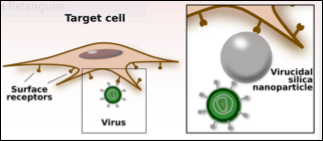
The design of new antiviral nanostructures must consider 2 goals: to effectively control viral infection; and to deal with the cytotoxicity related to the exposure of biological materials to structures in the submicrometer size range.
Silica is especially appealing when a functional, biocompatible, nontoxic, and inert material is desired. Further, it can be easily synthesized and engineered to present specific surface chemical properties or porous structures, allowing drug loading and release. Ligands grafted onto the surface of silica may be used to tune the physicochemical properties of particles, such as hydrophobicity and zeta-potential (electrical charge that a particle acquires due to the surrounding ions in solution), which strongly dictates the interaction of nanoparticles with cells and viruses.
To address the mechanism of antiviral nanoparticles action, Juliana Martins de Souza e Silva et al [1] have synthesized mesoporous silica nanoparticles presenting distinct surface groups, exhibiting distinct surface properties. They designed a series of experiments to evaluate their biocompatibility and their ability to inhibit the virus transduction of target cells and performed in vitro transduction assays on cell cultures, using a recombinant lentiviral vector engineered to express the GFP (green fluorescent protein) reporter gene. Each virus particle transduces a unique cell, which will then express the reporter gene, thus labeling the infected cell for fluorescence microscopy and flow cytometry. Transduction is mediated by virus envelope so two different preparations of a recombinant virus were generated: one that has the GFP lentiviral vector harboring a VSV-G envelope (vesicular stomatitis virus G glycoprotein); and another that has the GFP lentivector harboring an HIV-gp120 derived envelope.
Preparation and Characterization
Silica nanoparticles containing distinct surface groups were prepared. The particles were synthesized by hydrolysis and condensation of tetraethylorthosilicate (TEOS) under basic conditions. After that, they exhibited silanol groups Si−OH on their surfaces. Organic groups were grafted onto the surface of the silica nanoparticles when an organofunctional alkoxysilane was added to the reaction medium. After the addition of (3-aminopropyl) triethoxysilane (APTES), (3glycidyloxypropyl) trimethoxysilane (GPTMS), or trimethoxy (2-phenylethyl) silane (TMPES), the silica surface presented, respectively, aminopropyl, glycidyloxypropyl, or phenylethyl groups, in addition to −OH groups. They were named $\rm mSiO_{2}$-TEOS, $\rm mSiO_{2}$-APTES, $\rm mSiO_{2}$-GPTMS, and $\rm mSiO_{2}$-TMPES. The particles synthesized have a radius of a few hundred nanometers with an uniform spherical shape and smooth surfaces. No distinct surface features among the synthesized silica particles are observed by TEM images done at the Brazilian Nanotechnology National Laboratory (LNNano). Also, there is no apparent morphological difference among the nonfunctionalized particles and their functionalized counterparts. As expected, the estimated sizes slightly varied among the particles and the size distributions for $ \rm mSiO_{2}$-TEOS and for $ \rm mSiO_{2}$-APTES are centered at 382 and 354 nm, respectively. For $\rm mSiO_{2}$-GPTMS and $\rm mSiO_{2}$-TMPES, they are centered at 372 and 517 nm. From the SAXS results done at the LNLS, the particles exhibit mesoporous character and show the presence of periodical pores of nanometric dimensions. Zeta measurements were carried out in 40 mM KCl, PBS (phosphate buffered saline) and DMEM (cell culture solution): the values varied from −13.9 to +4.5, from −20.5 to −11.2 and from −22.8 to −17.5 mV in 40 mM KCl, PBS and DMEM (cell culture media) solutions. Significant differences in the overall nanoparticles charges highlight that the surface chemical modification produces materials with distinct colloidal properties.
Cytotoxicity and Inhibitory Potential of $\mathbf{mSiO_{2}}$ to Prevent Viral Transduction
Cell viability was monitored by the MTS (colorimetric assay for assessing cell metabolic activity) to estimate the cytotoxicity of the prepared particles and to define experimental conditions. In this way, cells were cultivated in the presence of different concentrations of nanoparticles. Cells cultivated in the absence of $\rm mSiO_{2}$ particles were used as the control experiment and they represented the total of healthy cells. Their response was normalized to 100% of cell metabolic activity.
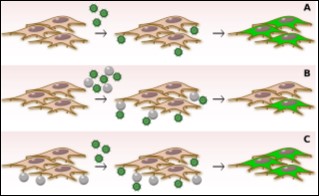
Figure 1. Schematics of the viral transduction process in the absence and presence of nanoparticles.
Partial results are illustrated in Fig. 1., describing the viral transduction process in the absence and presence of nanoparticles. Viral glycoproteins are represented by the spikes protruding from the viral particles (green spheres) and they bind to cells receptors shown on the cell surface.
(A)-Viral preparation is added to the cells. Viral particles bind to cells and are able to proceed with the transduction process. The viral genome (encoding GFP) is integrated into the cell genome and after 48 h of infection, cells express GFP and appear green under the appropriate laser light.
(B)-In the first assay performed with HIV-derived virus, $\rm mSiO_{2}$ particles were first incubated with the viral particles for 1 h, then added to the cells. The $\rm mSiO_{2}$ particles strongly interacting with the viral particles due to similar hydrophobicity/hydrophilicity properties will block the direct interaction between some viral particles and cells. After 48 h, some cells are transduced. (C)-In the second assay performed with HIV-derived virus, $\rm mSiO_{2}$ particles are left in contact with the cells for 1 h, then the viral preparation is added. In this situation, $\rm mSiO_{2}$ particles are not able to block all sites of interaction of viral particles and cells, and roughly all cells are infected after 48 h.
Because of the hydrophobicity/ hydrophilicity properties of their surfaces, $\rm mSiO_{2}$ particles may establish stronger interactions with a specific virus envelope with similar surface properties. At low concentrations, $\rm mSiO_{2}$ particles show no toxicity to the mammalian cells tested and are still able to reduce transduction of a recombinant vírus harboring VSV-G envelope. This envelope is largely used in basic research and was used in comparison with another recombinant lentivirus that harbors a HIV-gp120 envelope. The $\rm mSiO_{2}$ particles interactions with a specific virus envelope are the basis of the antiviral activity of the $\rm mSiO_{2}$ particles, as stronger virus-$\rm mSiO_{2}$ bonds disturb the attachment of cell receptors to the virus envelope, and the viral transduction ability is reduced up to 50%. The results demonstrate for the first time the antiviral activity of $\mathbf{mSiO_{2}}$ particles and allow proposing a mechanism of $ \mathbf{mSiO_{2}}$ particles antiviral action based on surface interactions between silica, cells and viruses. Thus, one could also explore the possibility of loading antiviral drugs into the mSiO2 particles mesopores.
Source: [1] Juliana Martins de Souza e Silva, Talita Diniz Melo Hanchuk, Murilo Izidoro Santos, Jörg Kobarg, Marcio Chaim Bajgelman, and Mateus Borba Cardoso. Viral Inhibition Mechanism Mediated by Surface-Modified Silica Nanoparticles. ACS Appl. Mater. Interfaces, 2016, 8 (26), pp 16564–16572. DOI: 10.1021/acsami.6b03342
The small interface roughness and polarization phenomena lead to a larger charge-carrier mobility and better operational stability.
New catalyst shows a potential for industrial applications requiring durability and high thermal stability.