
Research investigates cheaper alternatives to attenuate the emission of toxic gases
Despite the recent advances in the production of electric vehicles, and the future prospect of hydrogen-powered engines, the replacement of the fleet will take several years. In the meantime, the number of vehicles continues to grow. For that reason, not only public policies must be appropriate to this reality, but also innovative technologies to reduce the release of pollutants into the atmosphere must be developed.
The automotive catalytic converter is an equipment used in the exhaust of vehicles to reduce the emission of toxic gases. One of the reactions that occurs in this converter is the reduction of nitric oxide ($\rm NO$) by carbon monoxide ($\rm CO$) to form nitrogen gas ($\rm N_2$) and carbon dioxide ($\rm CO_2$). Most of the catalysts used in this reaction are based on noble metals, which are expensive and scarce, and, in some situations, promote the formation of nitrous oxide ($\rm N_2O$), which has a significant impact on the greenhouse effect and degradation of the ozone layer.
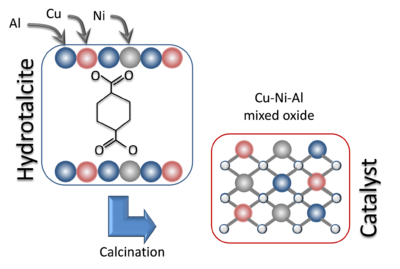
Schematic representation of the hydrotalcite precursor and the mixed oxide catalyst.
Therefore, Daniel Lopes et al. [1] investigated alternative catalysts based on copper ($\rm Cu$) and nickel ($\rm Ni$), for the reaction of $\rm NO$ reduction by $\rm CO$, evaluating its activity and its selectivity to produce $\rm N_2$. The catalysts were prepared from hydrotalcite precursors, containing the divalent $\rm Cu$ and $\rm Ni$ metals and the trivalent $\rm Al$ metal.
Different techniques were used investigate the properties of catalysts and precursors. In situ XANES was performed in the DXAS beamline of the Brazilian Synchrotron Light Laboratory (LNLS). With this technique, it was possible to determine the copper species present during the reaction to understand the phenomenon and the role of the catalyst in the reaction. In addition, techniques such as ex situ XANES and X-ray diffraction were also performed in the LNLS – in the DXAS and XPD beamlines respectively. From such analyses, it was possible to determine the composition of the crystalline phases and the oxidation states of the catalysts.
According to Fatima Zotin and Luz Amparo Palacio, coordinators of the research, the nickel and copper catalysts developed by the group have comparable, and in some cases superior, performance and selectivity, proving to be a promising alternative to noble metal catalysts.
Source: [1] Daniel Lopes, Fatima Zotin, Luz Amparo Palacio, Copper-nickel catalysts from hydrotalcite precursors: The performance in NO reduction by CO, Applied Catalysis B: Environmental 237 (2018) 327–338. DOI: 10.1016/j.apcatb.2018.06.007
First experimental report of a special optical layout dedicated to correct typical aberrations derived from large extraction ports in IR beamlines
New thermoelectric material shows great potential for wearable devices, embedded to clothing and accessories, and other flexible technologies