The proposed process could offer important technological and environmental advantages.
Growth in the use of high quality colored ceramics has stimulated research into the development of new classes of pigments with superior durability and color reproducibility, which can be produced using inexpensive, straightforward, and eco-friendly synthesis procedures.
At the same time, increasing efforts are aimed at solving the environmental problems related to the generation of waste by many industrial activities. Sustainable development needs to provide substantial reductions in waste generation, using strategies of prevention, reduction, recycling, and reuse. This has led to the concept of green manufacturing, also known as industrial symbiosis.
In this context, waste rich in toxic heavy metals can be used as raw material to produce ceramic pigments and catalysts. Waste from the tanning industry constitutes a major source of heavy metal pollutants, especially chromium. Globally, around 90% of this waste is discharged without any prior treatment, due to the high cost of disposal in landfills, and this can lead to harm to the environment. Waste from the leather tanning industry have higher concentrations of Cr (III), relative to Cr (VI). Trivalent chromium is much less toxic than hexavalent chromium, the latter being highly carcinogenic, as well as mutagenic. However, it is important to notice that Cr (III) can quickly convert to Cr (VI) under oxidizing conditions. It has been shown recently that the chlorination of water results in the oxidation of Cr (III) to Cr (VI) in only a few hours. In addition, when the concentration of Cr (III) exceeds a critical level, it becomes toxic and can decrease the activity of the immune system, causing greater structural damage in human erythrocyte membranes, compared to Cr (VI). Alteration of the permeability of biological membranes affects the functioning of receptors, ion channels, and enzymes, in addition to causing damage to DNA.
In a recent study, Graziele da Costa Cunha et al [1] have proposed a modified sol–gel route for the production of green and pink $ \rm Cr-Al_2O_3$ nanoparticles, employing solid and liquid tannery wastes together with NOM (natural organic material)-rich water. The synthesis involved preparing a solution of $ \rm Al_2Cl_3$ and the wastes in NOM-rich water. The solution was kept under stirring to form a gel that was then heated to 100 °C in order to eliminate water. This material was subsequently homogenized and calcined. The results indicated that single-phase chromium-doped α-alumina could be obtained using an initial solution pH of 4.0 and calcination at 1100 °C for 4h. The samples were characterized by various techniques: Thermal Analysis, Infrared Spectroscopy, X-Ray Diffraction, X-ray Absorption, X-Ray Fluorescence and Scanning Electron Microscopy.
Colorimetric analysis of the pigments was performed according to the CIE 1976 norm (quantitative links between the wavelengths and the physiological colors in the human vision). Chromaticity coordinates are used to define the quality of a pigment. According to previous studies a pigment is considered stable when the CIELAB coordinates show only minor differences between formulations. This is what it is observed in Fig 1.
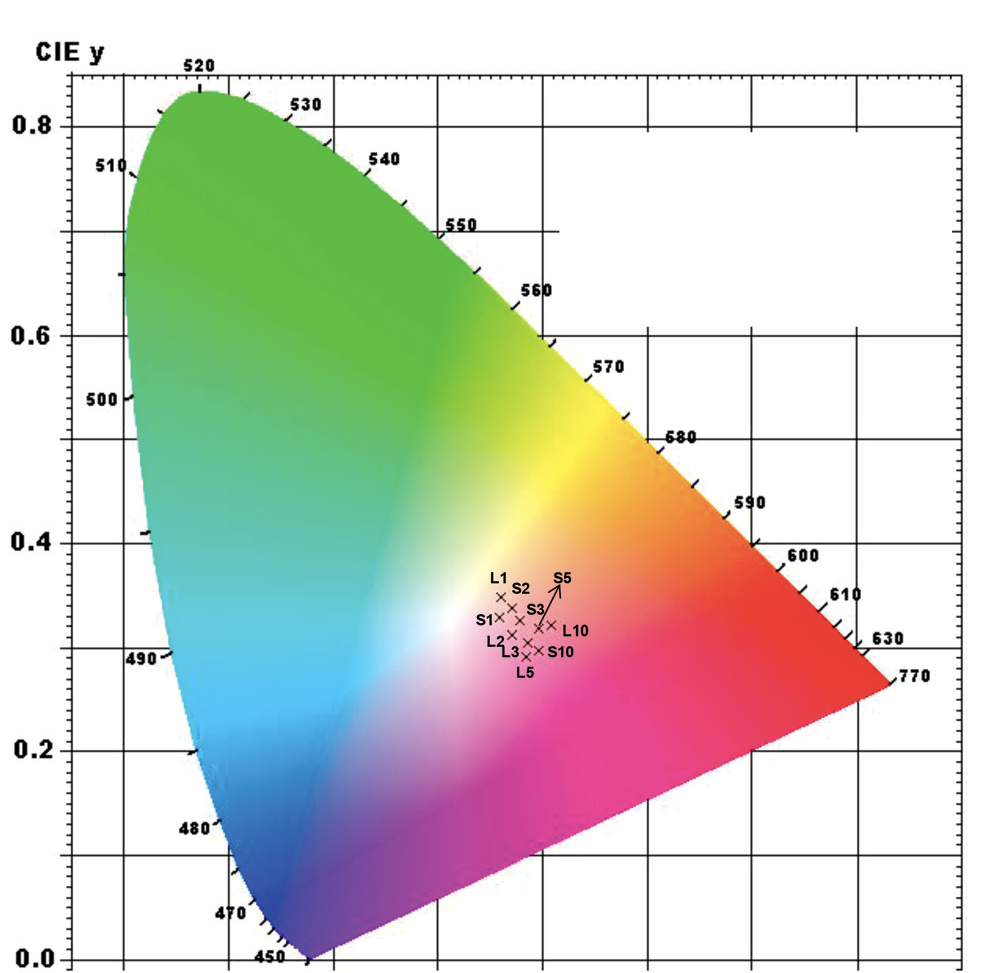
Figure 1: Chromaticity diagram (CIE 1931) of alumina doped with different concentrations of chromium ions derived from solid and liquid tannery wastes. The following nomenclature is used for the solid waste: S1 – $ Al_2O_3$/1%CrRS-EFS until S10 – $ Al_2O_3$/10%CrRS-EFS). For the liquid waste, the nomenclature used is: L1 – $ Al_2O_3$/1%CrRL-EFS until L10 – $ Al_2O_3$/10%CrRL-EFS).
Figure 1 shows the chromaticity diagram for alumina doped with different concentrations of recycled chromium ions, using the samples with lowest levels of impurities (RS-EFS and RL-EQS). The data represent the average of several readings for different pigment formulations synthesized using the same conditions. The results revealed a strong dependence of pigment color on the dopant concentration and pH used in the synthesis. The presence of the $ \rm Cr^{3+}$ oxidation state in all the samples studied was confirmed using X-ray absorption near-edge structure (XANES) spectroscopy done at the SXS beamline in the LNLS. The particle size of the samples was 39 ± 1 nm, as determined by FEG-SEM measurements. The catalytic tests demonstrated the potential of tannery wastes for use in the catalytic reduction of nitrophenol, with conversion rates between 49% and 98%. This shows that the proposed process could be used for the production of α-alumina pigments and catalysts using tannery wastes, offering important technological and environmental advantages.
Source:
[1] Graziele da Costa Cunha, Janaina Alves Peixoto, Daiane Requião de Souza, Luciane Pimenta Cruz Romão and Zélia Soares Macedo, Recycling of chromium wastes from the tanning industry to produce ceramic nanopigments. Green Chem., 2016, 18, 5342-5356. DOI: 10.1039/C6GC01562J
It is reported the magnetic characterization of the Fe3Ga4 intermetallic compound synthesized by the MFNN technique.
A detailed analysis highlights several structural complexities of the (BZCY72) perovskite.